Molecular insights of exercise therapy in disease prevention and treatment
- PMID: 38806473
- PMCID: PMC11133400
- DOI: 10.1038/s41392-024-01841-0
Molecular insights of exercise therapy in disease prevention and treatment
Abstract
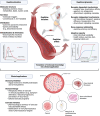
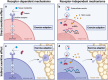
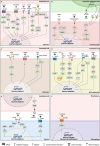
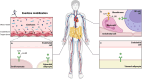
Despite substantial evidence emphasizing the pleiotropic benefits of exercise for the prevention and treatment of various diseases, the underlying biological mechanisms have not been fully elucidated. Several exercise benefits have been attributed to signaling molecules that are released in response to exercise by different tissues such as skeletal muscle, cardiac muscle, adipose, and liver tissue. These signaling molecules, which are collectively termed exerkines, form a heterogenous group of bioactive substances, mediating inter-organ crosstalk as well as structural and functional tissue adaption. Numerous scientific endeavors have focused on identifying and characterizing new biological mediators with such properties. Additionally, some investigations have focused on the molecular targets of exerkines and the cellular signaling cascades that trigger adaption processes. A detailed understanding of the tissue-specific downstream effects of exerkines is crucial to harness the health-related benefits mediated by exercise and improve targeted exercise programs in health and disease. Herein, we review the current in vivo evidence on exerkine-induced signal transduction across multiple target tissues and highlight the preventive and therapeutic value of exerkine signaling in various diseases. By emphasizing different aspects of exerkine research, we provide a comprehensive overview of (i) the molecular underpinnings of exerkine secretion, (ii) the receptor-dependent and receptor-independent signaling cascades mediating tissue adaption, and (iii) the clinical implications of these mechanisms in disease prevention and treatment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Knight, J. A. Physical inactivity: associated diseases and disorders. Ann. Clin. Lab. Sci.42, 320–337 (2012). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

