Mice with deficiency in Pcdh15, a gene associated with bipolar disorders, exhibit significantly elevated diurnal amplitudes of locomotion and body temperature
- PMID: 38806495
- PMCID: PMC11133426
- DOI: 10.1038/s41398-024-02952-6
Mice with deficiency in Pcdh15, a gene associated with bipolar disorders, exhibit significantly elevated diurnal amplitudes of locomotion and body temperature
Abstract
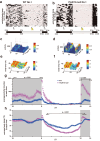
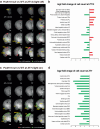
Genetic factors significantly affect the pathogenesis of psychiatric disorders. However, the specific pathogenic mechanisms underlying these effects are not fully understood. Recent extensive genomic studies have implicated the protocadherin-related 15 (PCDH15) gene in the onset of psychiatric disorders, such as bipolar disorder (BD). To further investigate the pathogenesis of these psychiatric disorders, we developed a mouse model lacking Pcdh15. Notably, although PCDH15 is primarily identified as the causative gene of Usher syndrome, which presents with visual and auditory impairments, our mice with Pcdh15 homozygous deletion (Pcdh15-null) did not exhibit observable structural abnormalities in either the retina or the inner ear. The Pcdh15-null mice showed very high levels of spontaneous motor activity which was too disturbed to perform standard behavioral testing. However, the Pcdh15 heterozygous deletion mice (Pcdh15-het) exhibited enhanced spontaneous locomotor activity, reduced prepulse inhibition, and diminished cliff avoidance behavior. These observations agreed with the symptoms observed in patients with various psychiatric disorders and several mouse models of psychiatric diseases. Specifically, the hyperactivity may mirror the manic episodes in BD. To obtain a more physiological, long-term quantification of the hyperactive phenotype, we implanted nano tag® sensor chips in the animals, to enable the continuous monitoring of both activity and body temperature. During the light-off period, Pcdh15-null exhibited elevated activity and body temperature compared with wild-type (WT) mice. However, we observed a decreased body temperature during the light-on period. Comprehensive brain activity was visualized using c-Fos mapping, which was assessed during the activity and temperature peak and trough. There was a stark contrast between the distribution of c-Fos expression in Pcdh15-null and WT brains during both the light-on and light-off periods. These results provide valuable insights into the neural basis of the behavioral and thermal characteristics of Pcdh15-deletion mice. Therefore, Pcdh15-deletion mice can be a novel model for BD with mania and other psychiatric disorders, with a strong genetic component that satisfies both construct and surface validity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures


References
MeSH terms
Substances
Grants and funding
- JP22tm0424222/Japan Agency for Medical Research and Development (AMED)
- JP21wm0425007/Japan Agency for Medical Research and Development (AMED)
- JP19dm0207075/Japan Agency for Medical Research and Development (AMED)
- JP19dm0207069/Japan Agency for Medical Research and Development (AMED)
- JP21dk0307103/Japan Agency for Medical Research and Development (AMED)
- JP20ek0109488/Japan Agency for Medical Research and Development (AMED)
- JP20dk0307099/Japan Agency for Medical Research and Development (AMED)
- JP22tm0424222/Japan Agency for Medical Research and Development (AMED)
- JP22gm1410011/Japan Agency for Medical Research and Development (AMED)
- JP23gm1910005/Japan Agency for Medical Research and Development (AMED)
- JP23ek0109678/Japan Agency for Medical Research and Development (AMED)
- JP19ak0101126/Japan Agency for Medical Research and Development (AMED)
- JP19ak0101113/Japan Agency for Medical Research and Development (AMED)
- 19K07384/MEXT | Japan Society for the Promotion of Science (JSPS)
- 22K07369/MEXT | Japan Society for the Promotion of Science (JSPS)
- 20K20602/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21H04815/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

