L-RNA aptamer-based CXCL12 inhibition combined with radiotherapy in newly-diagnosed glioblastoma: dose escalation of the phase I/II GLORIA trial
- PMID: 38806504
- PMCID: PMC11133480
- DOI: 10.1038/s41467-024-48416-9
L-RNA aptamer-based CXCL12 inhibition combined with radiotherapy in newly-diagnosed glioblastoma: dose escalation of the phase I/II GLORIA trial
Abstract
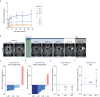
The chemokine CXCL12 promotes glioblastoma (GBM) recurrence after radiotherapy (RT) by facilitating vasculogenesis. Here we report outcomes of the dose-escalation part of GLORIA (NCT04121455), a phase I/II trial combining RT and the CXCL12-neutralizing aptamer olaptesed pegol (NOX-A12; 200/400/600 mg per week) in patients with incompletely resected, newly-diagnosed GBM lacking MGMT methylation. The primary endpoint was safety, secondary endpoints included maximum tolerable dose (MTD), recommended phase II dose (RP2D), NOX-A12 plasma levels, topography of recurrence, tumor vascularization, neurologic assessment in neuro-oncology (NANO), quality of life (QOL), median progression-free survival (PFS), 6-months PFS and overall survival (OS). Treatment was safe with no dose-limiting toxicities or treatment-related deaths. The MTD has not been reached and, thus, 600 mg per week of NOX-A12 was established as RP2D for the ongoing expansion part of the trial. With increasing NOX-A12 dose levels, a corresponding increase of NOX-A12 plasma levels was observed. Of ten patients enrolled, nine showed radiographic responses, four reached partial remission. All but one patient (90%) showed at best response reduced perfusion values in terms of relative cerebral blood volume (rCBV). The median PFS was 174 (range 58-260) days, 6-month PFS was 40.0% and the median OS 389 (144-562) days. In a post-hoc exploratory analysis of tumor tissue, higher frequency of CXCL12+ endothelial and glioma cells was significantly associated with longer PFS under NOX-A12. Our data imply safety of NOX-A12 and its efficacy signal warrants further investigation.
© 2024. The Author(s).
Conflict of interest statement
F.A.G. reports travel expenses, stocks and honoraria from TME Pharma AG related to this work; research grants and travel expenses from ELEKTA AB; grants, research grants, travel expenses and honoraria from Carl Zeiss Meditec AG; travel expenses and research grants from Varian Medical Systems, Inc.; travel expenses and/or honoraria from Bristol-Myers Squibb, Cureteq AG, Roche Pharma AG, MSD Sharp and Dohme GmbH, Siemens Healthineers AG, Varian Medical Systems, and AstraZeneca GmbH; non-financial support from Oncare GmbH and Opasca GmbH and patent US10857388B2 together with Carl Zeiss Meditec AG; all unrelated to this work. J.P.L. reports stocks and travel expenses from TME Pharma AG related to this work; travel expenses from Carl Zeiss Meditec AG, stocks and honoraria from Siemens Healthineers AG, and stocks from Bayer AG and BioNTech AG, all unrelated to this work. S.L. reports travel expenses from TME Pharma AG related to this work. C.S. has received speaker and/or advisory board honoraria from AbbVie, Bristol-Myers Squibb, HRA Pharma, Medac, Novocure, Roche, and Seagen not related to this work. E.S. reports travel expenses and honoraria for lectures from Carl Zeiss Meditec AG. C.O. reports travel support from Novocure; honoria by Horizon and Novocure and has received a Clinician Scientist Stipend of the University Medicine Essen Clinician Scientist Academy (UMEA) sponsored by the faculty of medicine and Deutsche Forschungsgemeinschaft (DFG). U.H. reports honoraria from Medac and Bayer AG, unrelated to this work. M.H. reports travel expenses, honoraria for webinars and research support (consumables) from TME Pharma AG related to this work. M.H. also reports honoraria from Bristol-Myers Squibb and Novartis unrelated to this work. A patent application related to biomarker identification has been filed by F.A.G., J.P.L., S.L., and M.H. (EP23000076.2; EP23000075.4). The remaining authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

