SARS-CoV-2-specific immune responses converge in kidney disease patients and controls with hybrid immunity
- PMID: 38806532
- PMCID: PMC11133345
- DOI: 10.1038/s41541-024-00886-0
SARS-CoV-2-specific immune responses converge in kidney disease patients and controls with hybrid immunity
Abstract
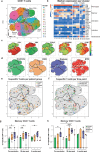
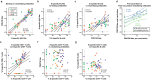
Healthy individuals with hybrid immunity, due to a SARS-CoV-2 infection prior to first vaccination, have stronger immune responses compared to those who were exclusively vaccinated. However, little is known about the characteristics of antibody, B- and T-cell responses in kidney disease patients with hybrid immunity. Here, we explored differences between kidney disease patients and controls with hybrid immunity after asymptomatic or mild coronavirus disease-2019 (COVID-19). We studied the kinetics, magnitude, breadth and phenotype of SARS-CoV-2-specific immune responses against primary mRNA-1273 vaccination in patients with chronic kidney disease or on dialysis, kidney transplant recipients, and controls with hybrid immunity. Although vaccination alone is less immunogenic in kidney disease patients, mRNA-1273 induced a robust immune response in patients with prior SARS-CoV-2 infection. In contrast, kidney disease patients with hybrid immunity develop SARS-CoV-2 antibody, B- and T-cell responses that are equally strong or stronger than controls. Phenotypic analysis showed that Spike (S)-specific B-cells varied between groups in lymph node-homing and memory phenotypes, yet S-specific T-cell responses were phenotypically consistent across groups. The heterogeneity amongst immune responses in hybrid immune kidney patients warrants further studies in larger cohorts to unravel markers of long-term protection that can be used for the design of targeted vaccine regimens.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Sanders JSF, et al. The RECOVAC Immune-response Study: The Immunogenicity, Tolerability, and Safety of COVID-19 Vaccination in Patients With Chronic Kidney Disease, on Dialysis, or Living With a Kidney Transplant. Transplantation. 2022;106:821–834. doi: 10.1097/TP.0000000000003983. - DOI - PMC - PubMed
Grants and funding
- 874735/EC | EU Framework Programme for Research and Innovation H2020 | H2020 European Institute of Innovation and Technology (H2020 The European Institute of Innovation and Technology)
- 101003589/EC | EU Framework Programme for Research and Innovation H2020 | H2020 European Institute of Innovation and Technology (H2020 The European Institute of Innovation and Technology)
- 109986/Nederlandse Organisatie voor Wetenschappelijk Onderzoek (Netherlands Organisation for Scientific Research)
- 10430072010002/ZonMw (Netherlands Organisation for Health Research and Development)
- 10430072110008/ZonMw (Netherlands Organisation for Health Research and Development)
LinkOut - more resources
Full Text Sources
Miscellaneous

