Polyetheretherketone bioactivity induced by farringtonite
- PMID: 38806564
- PMCID: PMC11133311
- DOI: 10.1038/s41598-024-61941-3
Polyetheretherketone bioactivity induced by farringtonite
Abstract
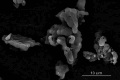
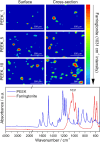
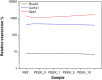
Polyetheretherketone (PEEK) is considered as an excellent biomaterial for bone grafting and connective tissue replacement. The clinical potential is, however, limited by its bioinertness, poor osteoconduction, and weak antibacterial activity. These disadvantages can be overcome by introducing suitable additives to produce mineral-polymer composites or coatings. In this work, a PEEK-based bioactive composite has been obtained by blending the polymer with magnesium phosphate (Mg3(PO4)2) particles in amounts ranging from 1 to 10 wt.% using the hot press technique. The obtained composite exhibited improved mechanical and physical properties, above the lower limits set for bone engineering applications. The tested grafts were found to not induce cytotoxicity. The presence of magnesium phosphate induced the mineralisation process with no adverse effects on the expression of the marker crucial for osteoblastic differentiation. The most promising results were observed in the grafts containing 1 wt.% of magnesium phosphate embedded within the PEEK matrix. The improved bioactivity of grafts, together with suitable physical-chemical and mechanical properties, indicate this composite as a promising orthopaedic implant material.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Tekin S, Cangül S, Adıgüzel Ö, Değer Y. Areas for use of PEEK material in dentistry. Int. Dent. Res. 2018;8:84–92. doi: 10.5577/intdentres.2018.vol8.no2.6. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

