Phenylacetic acid, an anti-vaginitis metabolite produced by the vaginal symbiotic bacterium Chryseobacterium gleum
- PMID: 38806600
- PMCID: PMC11133378
- DOI: 10.1038/s41598-024-62947-7
Phenylacetic acid, an anti-vaginitis metabolite produced by the vaginal symbiotic bacterium Chryseobacterium gleum
Abstract
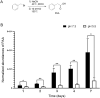
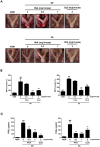
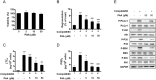
The human microbiome contains genetic information that regulates metabolic processes in response to host health and disease. While acidic vaginal pH is maintained in normal conditions, the pH level increases in infectious vaginitis. We propose that this change in the vaginal environment triggers the biosynthesis of anti-vaginitis metabolites. Gene expression levels of Chryseobacterium gleum, a vaginal symbiotic bacterium, were found to be affected by pH changes. The distinctive difference in the metabolic profiles between two C. gleum cultures incubated under acidic and neutral pH conditions was suggested to be an anti-vaginitis molecule, which was identified as phenylacetic acid (PAA) by spectroscopic data analysis. The antimicrobial activity of PAA was evaluated in vitro, showing greater toxicity toward Gardnerella vaginalis and Candida albicans, two major vaginal pathogens, relative to commensal Lactobacillus spp. The activation of myeloperoxidase, prostaglandin E2, and nuclear factor-κB, and the expression of cyclooxygenase-2 were reduced by an intravaginal administration of PAA in the vaginitis mouse model. In addition, PAA displayed the downregulation of mast cell activation. Therefore, PAA was suggested to be a messenger molecule that mediates interactions between the human microbiome and vaginal health.
Keywords: Chryseobacterium gleum; Human microbiome; Phenylacetic acid; Vaginitis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Wang B, Yao M, Lv L, Ling Z, Li L. The human microbiota in health and disease. Engineering (Beijing) 2017;3:71–82. doi: 10.1016/J.ENG.2017.01.008. - DOI
-
- Proctor L, LoTempio J, Marquitz A, Daschner P, Xi D, Flores R, Brown L, Ranallo R, Maruvada P, Regan K, Lunsford RD, Reddy M, Caler L. A review of 10 years of human microbiome research activities at the US National Institutes of Health, Fiscal Years 2007–2016. Microbiome. 2019;7:31. doi: 10.1186/s40168-019-0620-y. - DOI - PMC - PubMed
-
- Donia MS, Cimermancic P, Schulze CJ, Brown LCW, Martin J, Mitreva M, Clardy J, Linington RG, Fischbach MA. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell. 2014;158:1402–1414. doi: 10.1016/j.cell.2014.08.032. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

