Mycobacterium tuberculosis produces D-serine under hypoxia to limit CD8+ T cell-dependent immunity in mice
- PMID: 38806671
- PMCID: PMC11222154
- DOI: 10.1038/s41564-024-01701-1
Mycobacterium tuberculosis produces D-serine under hypoxia to limit CD8+ T cell-dependent immunity in mice
Abstract
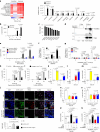
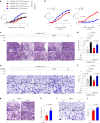
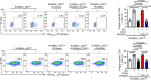
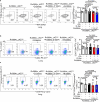
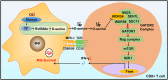
Adaptation to hypoxia is a major challenge for the survival of Mycobacterium tuberculosis (Mtb) in vivo. Interferon (IFN)-γ-producing CD8+ T cells contribute to control of Mtb infection, in part by promoting antimicrobial activities of macrophages. Whether Mtb counters these responses, particularly during hypoxic conditions, remains unknown. Using metabolomic, proteomic and genetic approaches, here we show that Mtb induced Rv0884c (SerC), an Mtb phosphoserine aminotransferase, to produce D-serine. This activity increased Mtb pathogenesis in mice but did not directly affect intramacrophage Mtb survival. Instead, D-serine inhibited IFN-γ production by CD8+ T cells, which indirectly reduced the ability of macrophages to restrict Mtb upon co-culture. Mechanistically, D-serine interacted with WDR24 and inhibited mTORC1 activation in CD8+ T cells. This decreased T-bet expression and reduced IFN-γ production by CD8+ T cells. Our findings suggest an Mtb evasion mechanism where pathogen metabolic adaptation to hypoxia leads to amino acid-dependent suppression of adaptive anti-TB immunity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

