Microbial polyphenol metabolism is part of the thawing permafrost carbon cycle
- PMID: 38806673
- PMCID: PMC11153144
- DOI: 10.1038/s41564-024-01691-0
Microbial polyphenol metabolism is part of the thawing permafrost carbon cycle
Abstract
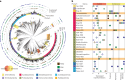
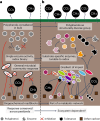
With rising global temperatures, permafrost carbon stores are vulnerable to microbial degradation. The enzyme latch theory states that polyphenols should accumulate in saturated peatlands due to diminished phenol oxidase activity, inhibiting resident microbes and promoting carbon stabilization. Pairing microbiome and geochemical measurements along a permafrost thaw-induced saturation gradient in Stordalen Mire, a model Arctic peatland, we confirmed a negative relationship between phenol oxidase expression and saturation but failed to support other trends predicted by the enzyme latch. To inventory alternative polyphenol removal strategies, we built CAMPER, a gene annotation tool leveraging polyphenol enzyme knowledge gleaned across microbial ecosystems. Applying CAMPER to genome-resolved metatranscriptomes, we identified genes for diverse polyphenol-active enzymes expressed by various microbial lineages under a range of redox conditions. This shifts the paradigm that polyphenols stabilize carbon in saturated soils and highlights the need to consider both oxic and anoxic polyphenol metabolisms to understand carbon cycling in changing ecosystems.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Hugelius G, et al. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences. 2014;11:6573–6593. doi: 10.5194/bg-11-6573-2014. - DOI
-
- Schuur EAG, et al. Permafrost and climate change: carbon cycle feedbacks from the warming arctic. Annu. Rev. Environ. Resour. 2022;47:343–371. doi: 10.1146/annurev-environ-012220-011847. - DOI
-
- Turetsky MR, et al. Carbon release through abrupt permafrost thaw. Nat. Geosci. 2020;13:138–143. doi: 10.1038/s41561-019-0526-0. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

