XPD stalled on cross-linked DNA provides insight into damage verification
- PMID: 38806694
- PMCID: PMC11479942
- DOI: 10.1038/s41594-024-01323-5
XPD stalled on cross-linked DNA provides insight into damage verification
Abstract
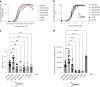
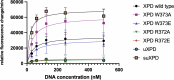
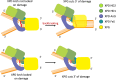
The superfamily 2 helicase XPD is a central component of the general transcription factor II H (TFIIH), which is essential for transcription and nucleotide excision DNA repair (NER). Within these two processes, the helicase function of XPD is vital for NER but not for transcription initiation, where XPD acts only as a scaffold for other factors. Using cryo-EM, we deciphered one of the most enigmatic steps in XPD helicase action: the active separation of double-stranded DNA (dsDNA) and its stalling upon approaching a DNA interstrand cross-link, a highly toxic form of DNA damage. The structure shows how dsDNA is separated and reveals a highly unusual involvement of the Arch domain in active dsDNA separation. Combined with mutagenesis and biochemical analyses, we identified distinct functional regions important for helicase activity. Surprisingly, those areas also affect core TFIIH translocase activity, revealing a yet unencountered function of XPD within the TFIIH scaffold. In summary, our data provide a universal basis for NER bubble formation, XPD damage verification and XPG incision.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









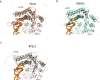
References
-
- Hoeijmakers, J. H. Genome maintenance mechanisms for preventing cancer. Nature411, 366–374 (2001). - PubMed
-
- Gillet, L. C. & Scharer, O. D. Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem. Rev.106, 253–276 (2006). - PubMed
-
- Scharer, O. D. Chemistry and biology of DNA repair. Angew. Chem. Int. Ed. Engl.42, 2946–2974 (2003). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

