ROBIN: a randomised, double-masked, placebo-controlled Phase IIa study of the AOC3 inhibitor BI 1467335 in diabetic retinopathy
- PMID: 38806700
- PMCID: PMC11226676
- DOI: 10.1038/s41433-024-03017-0
ROBIN: a randomised, double-masked, placebo-controlled Phase IIa study of the AOC3 inhibitor BI 1467335 in diabetic retinopathy
Abstract
Objective: To evaluate the safety and efficacy of BI 1467335 in patients with non-proliferative diabetic retinopathy (NPDR).
Methods: ROBIN is a Phase IIa, double-masked, randomised, placebo-controlled study (NCT03238963). Patients with NPDR and without centre-involved diabetic macular oedema were included; all had a best corrected visual acuity letter score of ≥70 Early Treatment Diabetic Retinopathy Study letters in the study eye at screening. Patients received oral BI 1467335 10 mg or placebo once daily for 12 weeks. Post-treatment follow-up was 12 weeks. The primary endpoint was the proportion of patients over the 24 weeks with ocular adverse events (AEs). Secondary endpoints were the proportion of patients with ≥2-step improvement from baseline in DRSS severity level at Week 12 and the proportion of patients with non-ocular AEs at 24 weeks.
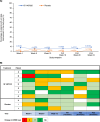
Results: Seventy-nine patients entered the study (BI 1467335, n = 40; placebo, n = 39). The proportion of patients with ocular AEs over 24 weeks was greater in the BI 1467335 versus the placebo group (35.0% vs 23.1%, respectively). Treatment-related AEs were reported for similar numbers of patients in the placebo and BI 1467335 group (7.7% vs 7.5%, respectively). At Week 12, 5.7% (n = 2) of patients in the BI 1467335 group had a 2-step improvement in DRSS severity level from baseline, compared with 0% in the placebo group.
Conclusions: BI 1467335 was well tolerated by patients with NPDR. There was a high variability in DRSS levels for individual patients over time, with no clear efficacy signal.
© 2024. The Author(s).
Conflict of interest statement
QDN declares funding from Boehringer Ingelheim, Genentech, Gilead, Regeneron and Santen, and consulting for Genentech, Novartis, Regeneron and Santen. JPE declares that he is a consultant for Adverum, Aerpio, Alcon, Allergan, Boehringer Ingelheim, Genentech, Leica, Novartis, Regeneron, Roche, Stealth and Zeiss, and receives grant support from Adverum, Alcon, Allergan, Boehringer Ingelheim, Novartis, Roche, Regeneron, Stealth and Zeiss. DSB declares that he is a consultant with Acucela, Alimera Sciences, Allegro, Bayer, EyePoint Pharmaceuticals, ONL Therapeutics, Oxurion and Takeda; is a consultant and researcher with Adverum, Aerpio, Allergan, Apellis, Boehringer Ingelheim, Chengdu Kanghong, Clearside Biomedical, Genentech/Roche, Kodiak, Novartis, Regeneron, REGENXBIO, Roche and Santen; and is a researcher with Aerie Pharmaceuticals, Gemini Therapeutics, Graybug Vision, IONIS Pharmaceuticals, Neurotech, Opthea and Outlook Therapeutics. XJ and AG declare that they are employees of Boehringer Ingelheim. MSE declares that he is an employee of Alexion Pharmaceuticals, but that this work was performed prior to Alexion employment and is not endorsed by Alexion; at the time of project initiation, he was an employee of Boehringer Ingelheim.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

