Circadian control of tumor immunosuppression affects efficacy of immune checkpoint blockade
- PMID: 38806707
- PMCID: PMC11374317
- DOI: 10.1038/s41590-024-01859-0
Circadian control of tumor immunosuppression affects efficacy of immune checkpoint blockade
Abstract
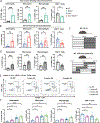
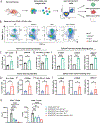
The circadian clock is a critical regulator of immunity, and this circadian control of immune modulation has an essential function in host defense and tumor immunosurveillance. Here we use a single-cell RNA sequencing approach and a genetic model of colorectal cancer to identify clock-dependent changes to the immune landscape that control the abundance of immunosuppressive cells and consequent suppression of cytotoxic CD8+ T cells. Of these immunosuppressive cell types, PD-L1-expressing myeloid-derived suppressor cells (MDSCs) peak in abundance in a rhythmic manner. Disruption of the epithelial cell clock regulates the secretion of cytokines that promote heightened inflammation, recruitment of neutrophils and the subsequent development of MDSCs. We also show that time-of-day anti-PD-L1 delivery is most effective when synchronized with the abundance of immunosuppressive MDSCs. Collectively, these data indicate that circadian gating of tumor immunosuppression informs the timing and efficacy of immune checkpoint inhibitors.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing Interests Statement:
The authors declare no competing financial interests.
Figures




References
-
- Ben-Aharon I et al. Early-Onset Cancer in the Gastrointestinal Tract Is on the Rise—Evidence and Implications. Cancer Discovery 13, 538–551 (2023). - PubMed
-
- Sinicrope FA Increasing Incidence of Early-Onset Colorectal Cancer. New England Journal of Medicine 386, 1547–1558 (2022). - PubMed
-
- Siegel RL, Miller KD, Wagle NS & Jemal A Cancer statistics, 2023. CA: A Cancer Journal for Clinicians 73, 17–48 (2023). - PubMed
-
- Tsalic M, Bar-Sela G, Beny A, Visel B & Haim N Severe Toxicity Related to the 5-Fluorouracil/Leucovorin Combination (The Mayo Clinic Regimen): A Prospective Study in Colorectal Cancer Patients. American Journal of Clinical Oncology 26, 103 (2003). - PubMed
MeSH terms
Substances
Grants and funding
- R01 CA244519/CA/NCI NIH HHS/United States
- R01CA259370/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- R37CA266042/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- R01 CA276470/CA/NCI NIH HHS/United States
- DP1 DK130640/DK/NIDDK NIH HHS/United States
- DGE1839285/National Science Foundation (NSF)
- T32 CA009054/CA/NCI NIH HHS/United States
- R37 CA266042/CA/NCI NIH HHS/United States
- DP1DK130640/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- R01CA276470/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- P30 CA062203/CA/NCI NIH HHS/United States
- F31 CA287992/CA/NCI NIH HHS/United States
- R01 CA259370/CA/NCI NIH HHS/United States
- R01CA244519/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- T32CA009054/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- F31 AR083279/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

