Nociceptor-immune interactomes reveal insult-specific immune signatures of pain
- PMID: 38806708
- PMCID: PMC11224023
- DOI: 10.1038/s41590-024-01857-2
Nociceptor-immune interactomes reveal insult-specific immune signatures of pain
Abstract
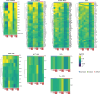
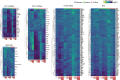
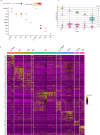
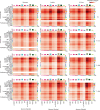
Inflammatory pain results from the heightened sensitivity and reduced threshold of nociceptor sensory neurons due to exposure to inflammatory mediators. However, the cellular and transcriptional diversity of immune cell and sensory neuron types makes it challenging to decipher the immune mechanisms underlying pain. Here we used single-cell transcriptomics to determine the immune gene signatures associated with pain development in three skin inflammatory pain models in mice: zymosan injection, skin incision and ultraviolet burn. We found that macrophage and neutrophil recruitment closely mirrored the kinetics of pain development and identified cell-type-specific transcriptional programs associated with pain and its resolution. Using a comprehensive list of potential interactions mediated by receptors, ligands, ion channels and metabolites to generate injury-specific neuroimmune interactomes, we also uncovered that thrombospondin-1 upregulated by immune cells upon injury inhibited nociceptor sensitization. This study lays the groundwork for identifying the neuroimmune axes that modulate pain in diverse disease contexts.
© 2024. The Author(s).
Conflict of interest statement
C.J.W. is a founder of Nocion Therapeutics, Quralis and Blackbox Bio, an SAB member of Lundbeck, Axonis and Tafalgie Therapeutics, and a consultant for GSK. P.K.S. is a member of the Board of Directors of Glencoe Software and Applied Biomath, a member of the SAB for RareCyte, NanoString and Montai Health, and a consultant for Merck. The remaining authors declare no competing interests.
Figures




References
-
- Jain A, Hakim S, Woolf CJ. Unraveling the plastic peripheral neuroimmune interactome. J. Immunol. 2020;204:257–263. - PubMed
MeSH terms
Substances
Grants and funding
- HR0011-19-2-0022/United States Department of Defense | Defense Advanced Research Projects Agency (DARPA)
- P50 HD105351/HD/NICHD NIH HHS/United States
- R35 NS105076/NS/NINDS NIH HHS/United States
- R35NS105076/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- W911NF-20-1-0255/United States Department of Defense | Defense Advanced Research Projects Agency (DARPA)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

