A systematic review on the culture methods and applications of 3D tumoroids for cancer research and personalized medicine
- PMID: 38806997
- PMCID: PMC11850459
- DOI: 10.1007/s13402-024-00960-8
A systematic review on the culture methods and applications of 3D tumoroids for cancer research and personalized medicine
Abstract
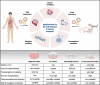
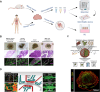
Cancer is a highly heterogeneous disease, and thus treatment responses vary greatly between patients. To improve therapy efficacy and outcome for cancer patients, more representative and patient-specific preclinical models are needed. Organoids and tumoroids are 3D cell culture models that typically retain the genetic and epigenetic characteristics, as well as the morphology, of their tissue of origin. Thus, they can be used to understand the underlying mechanisms of cancer initiation, progression, and metastasis in a more physiological setting. Additionally, co-culture methods of tumoroids and cancer-associated cells can help to understand the interplay between a tumor and its tumor microenvironment. In recent years, tumoroids have already helped to refine treatments and to identify new targets for cancer therapy. Advanced culturing systems such as chip-based fluidic devices and bioprinting methods in combination with tumoroids have been used for high-throughput applications for personalized medicine. Even though organoid and tumoroid models are complex in vitro systems, validation of results in vivo is still the common practice. Here, we describe how both animal- and human-derived tumoroids have helped to identify novel vulnerabilities for cancer treatment in recent years, and how they are currently used for precision medicine.
Keywords: 3D models; Bioprinting; Cancer; Co-culture; Fluidic devices; Organoids; Precision medicine; Preclinical models; Tumoroids.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Copyright: Where specified, figures were reproduced from cited publications with permission of the respective journals, or were adapted from cited publications under the Creative Commons Attribution 4.0 International ( https://creativecommons.org/licenses/by/4.0/ . Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures

Similar articles
-
Optimized scaffold-free human 3D adipose tissue organoid culture for obesity and disease modeling.SLAS Discov. 2025 Mar;31:100218. doi: 10.1016/j.slasd.2025.100218. Epub 2025 Jan 25. SLAS Discov. 2025. PMID: 39870353
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Recent Advances in Graphene Oxide-Based on Organoid Culture as Disease Model and Cell Behavior - A Systematic Literature Review.Int J Nanomedicine. 2024 Jun 19;19:6201-6228. doi: 10.2147/IJN.S455940. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38911499 Free PMC article.
-
Innovative organ-on-a-chip platforms for exploring tumorigenesis and therapy in head and neck cancer.J Transl Med. 2025 Jul 16;23(1):798. doi: 10.1186/s12967-025-06824-5. J Transl Med. 2025. PMID: 40671128 Free PMC article. Review.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
Cited by
-
Perfusion-based ex vivo culture of frozen ovarian cancer tissues with preserved tumor microenvironment.NPJ Precis Oncol. 2025 May 23;9(1):152. doi: 10.1038/s41698-025-00941-6. NPJ Precis Oncol. 2025. PMID: 40410344 Free PMC article.
-
Modeling the Bone Marrow Niche in Multiple Myeloma: From 2D Cultures to 3D Systems.Int J Mol Sci. 2025 Jun 27;26(13):6229. doi: 10.3390/ijms26136229. Int J Mol Sci. 2025. PMID: 40649999 Free PMC article. Review.
-
The atypical KRASQ22K mutation directs TGF-β response towards partial epithelial-to-mesenchymal transition in patient-derived colorectal cancer tumoroids.Mol Oncol. 2025 Aug;19(8):2212-2232. doi: 10.1002/1878-0261.70014. Epub 2025 Mar 11. Mol Oncol. 2025. PMID: 40066744 Free PMC article.
-
Medulloblastoma: biology and immunotherapy.Front Immunol. 2025 Jul 3;16:1602930. doi: 10.3389/fimmu.2025.1602930. eCollection 2025. Front Immunol. 2025. PMID: 40677711 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

