MnO2 and roflumilast-loaded probiotic membrane vesicles mitigate experimental colitis by synergistically augmenting cAMP in macrophage
- PMID: 38807127
- PMCID: PMC11131305
- DOI: 10.1186/s12951-024-02558-6
MnO2 and roflumilast-loaded probiotic membrane vesicles mitigate experimental colitis by synergistically augmenting cAMP in macrophage
Abstract
Background: Ulcerative colitis (UC) is one chronic and relapsing inflammatory bowel disease. Macrophage has been reputed as one trigger for UC. Recently, phosphodiesterase 4 (PDE4) inhibitors, for instance roflumilast, have been regarded as one latent approach to modulating macrophage in UC treatment. Roflumilast can decelerate cyclic adenosine monophosphate (cAMP) degradation, which impedes TNF-α synthesis in macrophage. However, roflumilast is devoid of macrophage-target and consequently causes some unavoidable adverse reactions, which restrict the utilization in UC.
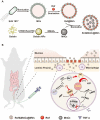
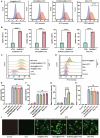
Results: Membrane vesicles (MVs) from probiotic Escherichia coli Nissle 1917 (EcN 1917) served as a drug delivery platform for targeting macrophage. As model drugs, roflumilast and MnO2 were encapsulated in MVs (Rof&MnO2@MVs). Roflumilast inhibited cAMP degradation via PDE4 deactivation and MnO2 boosted cAMP generation by activating adenylate cyclase (AC). Compared with roflumilast, co-delivery of roflumilast and MnO2 apparently produced more cAMP and less TNF-α in macrophage. Besides, Rof&MnO2@MVs could ameliorate colitis in mouse model and regulate gut microbe such as mitigating pathogenic Escherichia-Shigella and elevating probiotic Akkermansia.
Conclusions: A probiotic-based nanoparticle was prepared for precise codelivery of roflumilast and MnO2 into macrophage. This biomimetic nanoparticle could synergistically modulate cAMP in macrophage and ameliorate experimental colitis.
Keywords: Macrophage; Membrane vesicles; MnO2; Roflumilast; Ulcerative colitis; cAMP.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

