Composite outcome measures in high-impact critical care randomised controlled trials: a systematic review
- PMID: 38807143
- PMCID: PMC11134769
- DOI: 10.1186/s13054-024-04967-3
Composite outcome measures in high-impact critical care randomised controlled trials: a systematic review
Abstract
Background: The use of composite outcome measures (COM) in clinical trials is increasing. Whilst their use is associated with benefits, several limitations have been highlighted and there is limited literature exploring their use within critical care. The primary aim of this study was to evaluate the use of COM in high-impact critical care trials, and compare study parameters (including sample size, statistical significance, and consistency of effect estimates) in trials using composite versus non-composite outcomes.
Methods: A systematic review of 16 high-impact journals was conducted. Randomised controlled trials published between 2012 and 2022 reporting a patient important outcome and involving critical care patients, were included.
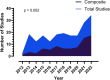
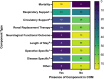
Results: 8271 trials were screened, and 194 included. 39.1% of all trials used a COM and this increased over time. Of those using a COM, only 52.6% explicitly described the outcome as composite. The median number of components was 2 (IQR 2-3). Trials using a COM recruited fewer participants (409 (198.8-851.5) vs 584 (300-1566, p = 0.004), and their use was not associated with increased rates of statistical significance (19.7% vs 17.8%, p = 0.380). Predicted effect sizes were overestimated in all but 6 trials. For studies using a COM the effect estimates were consistent across all components in 43.4% of trials. 93% of COM included components that were not patient important.
Conclusions: COM are increasingly used in critical care trials; however effect estimates are frequently inconsistent across COM components confounding outcome interpretations. The use of COM was associated with smaller sample sizes, and no increased likelihood of statistically significant results. Many of the limitations inherent to the use of COM are relevant to critical care research.
Keywords: Critical care; Critical care outcomes; Outcome assessment; Randomized controlled trials as topic.
© 2024. The Author(s).
Conflict of interest statement
No potential conflicts of interest relevant to this article were reported however AU reports grants (paid to Monash University) from NHMRC Australia, MRFF Australia and the Department of Health, Commonwealth of Australia that are unrelated to this work and the receipt of in-kind support (trial consumables) from Integra Lifesciences for a project outside of the current work.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

