Biological effects of corticosteroids on pneumococcal pneumonia in Mice-translational significance
- PMID: 38807178
- PMCID: PMC11134653
- DOI: 10.1186/s13054-024-04956-6
Biological effects of corticosteroids on pneumococcal pneumonia in Mice-translational significance
Abstract
Background: Streptococcus pneumoniae is the most common bacterial cause of community acquired pneumonia and the acute respiratory distress syndrome (ARDS). Some clinical trials have demonstrated a beneficial effect of corticosteroid therapy in community acquired pneumonia, COVID-19, and ARDS, but the mechanisms of this benefit remain unclear. The primary objective of this study was to investigate the effects of corticosteroids on the pulmonary biology of pneumococcal pneumonia in a mouse model. A secondary objective was to identify shared transcriptomic features of pneumococcal pneumonia and steroid treatment in the mouse model and clinical samples.
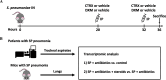
Methods: We carried out comprehensive physiologic, biochemical, and histological analyses in mice to identify the mechanisms of lung injury in Streptococcus pneumoniae with and without adjunctive steroid therapy. We also studied lower respiratory tract gene expression from a cohort of 15 mechanically ventilated patients (10 with Streptococcus pneumoniae and 5 controls) to compare with the transcriptional studies in the mice.
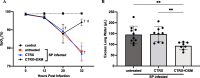
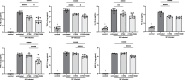
Results: In mice with pneumonia, dexamethasone in combination with ceftriaxone reduced (1) pulmonary edema formation, (2) alveolar protein permeability, (3) proinflammatory cytokine release, (4) histopathologic lung injury score, and (5) hypoxemia but did not increase bacterial burden. Transcriptomic analyses identified effects of steroid therapy in mice that were also observed in the clinical samples.
Conclusions: In combination with appropriate antibiotic therapy in mice, treatment of pneumococcal pneumonia with steroid therapy reduced hypoxemia, pulmonary edema, lung permeability, and histologic criteria of lung injury, and also altered inflammatory responses at the protein and gene expression level. The transcriptional studies in patients suggest that the mouse model replicates some of the features of pneumonia in patients with Streptococcus pneumoniae and steroid treatment. Overall, these studies provide evidence for the mechanisms that may explain the beneficial effects of glucocorticoid therapy in patients with community acquired pneumonia from Streptococcus Pneumoniae.
Keywords: Acute respiratory distress syndrome; Glucocorticoids; Pneumonia; Streptococcal infections.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Update of
-
Biological Effects of Corticosteroids on Pneumococcal Pneumonia in Mice and Humans.Res Sq [Preprint]. 2024 Feb 21:rs.3.rs-3962861. doi: 10.21203/rs.3.rs-3962861/v1. Res Sq. 2024. Update in: Crit Care. 2024 May 29;28(1):185. doi: 10.1186/s13054-024-04956-6. PMID: 38464245 Free PMC article. Updated. Preprint.
References
-
- Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, Albertson SB, Deshpande A, Farag T, Abebe Z, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–1210. doi: 10.1016/S1473-3099(18)30310-4. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

