Protein kinase CK2α is overexpressed in classical hodgkin lymphoma, regulates key signaling pathways, PD-L1 and may represent a new target for therapy
- PMID: 38807597
- PMCID: PMC11130512
- DOI: 10.3389/fimmu.2024.1393485
Protein kinase CK2α is overexpressed in classical hodgkin lymphoma, regulates key signaling pathways, PD-L1 and may represent a new target for therapy
Abstract
Introduction: In classical Hodgkin lymphoma (cHL), the survival of neoplastic cells is mediated by the activation of NF-κB, JAK/STAT and PI3K/Akt signaling pathways. CK2 is a highly conserved serine/threonine kinase, consisting of two catalytic (α) and two regulatory (β) subunits, which is involved in several cellular processes and both subunits were found overexpressed in solid tumors and hematologic malignancies.
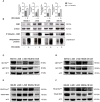
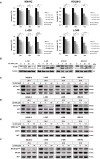
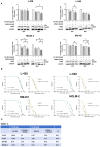
Methods and results: Biochemical analyses and in vitro assays showed an impaired expression of CK2 subunits in cHL, with CK2α being overexpressed and a decreased expression of CK2β compared to normal B lymphocytes. Mechanistically, CK2β was found to be ubiquitinated in all HL cell lines and consequently degraded by the proteasome pathway. Furthermore, at basal condition STAT3, NF-kB and AKT are phosphorylated in CK2-related targets, resulting in constitutive pathways activation. The inhibition of CK2 with CX-4945/silmitasertib triggered the de-phosphorylation of NF-κB-S529, STAT3-S727, AKT-S129 and -S473, leading to cHL cell lines apoptosis. Moreover, CX-4945/silmitasertib was able to decrease the expression of the immuno-checkpoint CD274/PD-L1 but not of CD30, and to synergize with monomethyl auristatin E (MMAE), the microtubule inhibitor of brentuximab vedotin.
Conclusions: Our data point out a pivotal role of CK2 in the survival and the activation of key signaling pathways in cHL. The skewed expression between CK2α and CK2β has never been reported in other lymphomas and might be specific for cHL. The effects of CK2 inhibition on PD-L1 expression and the synergistic combination of CX-4945/silmitasertib with MMAE pinpoints CK2 as a high-impact target for the development of new therapies for cHL.
Keywords: CK2; MMAE; PD-L1; anti-CD30; classical hodgkin lymphoma.
Copyright © 2024 Ruggeri, Frezzato, Mouawad, Pizzi, Scarmozzino, Capasso, Trimarco, Quotti Tubi, Cellini, Cavarretta, Ruocco, Serafin, Angotzi, Danesin, Manni, Facco, Piazza, Trentin and Visentin.
Conflict of interest statement
AV and LT participated to scientific board organized and received travel grant by Takeda. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- Cellini A, Scarmozzino F, Angotzi F, Ruggeri E, Dei Tos AP, Trentin L, et al. . Tackling the dysregulated immune-checkpoints in classical Hodgkin lymphoma: bidirectional regulations between the microenvironment and Hodgkin/Reed-Sternberg cells. Front Oncol. (2023) 13:1203470. doi: 10.3389/fonc.2023.1203470 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

