Transient heat stress protects from severe endothelial damage and dysfunction during prolonged experimental ex-vivo lung perfusion
- PMID: 38807604
- PMCID: PMC11130382
- DOI: 10.3389/fimmu.2024.1390026
Transient heat stress protects from severe endothelial damage and dysfunction during prolonged experimental ex-vivo lung perfusion
Abstract
Introduction: The pulmonary endothelium is the primary target of lung ischemia-reperfusion injury leading to primary graft dysfunction after lung transplantation. We hypothesized that treating damaged rat lungs by a transient heat stress during ex-vivo lung perfusion (EVLP) to elicit a pulmonary heat shock response could protect the endothelium from severe reperfusion injury.
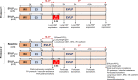
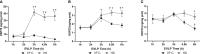
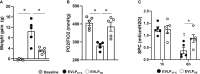
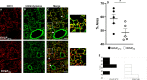
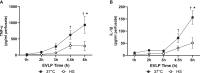
Methods: Rat lungs damaged by 1h warm ischemia were reperfused on an EVLP platform for up to 6h at a constant temperature (T°) of 37°C (EVLP37°C group), or following a transient heat stress (HS) at 41.5°C from 1 to 1.5h of EVLP (EVLPHS group). A group of lungs exposed to 1h EVLP only (pre-heating conditions) was added as control (Baseline group). In a first protocol, we measured lung heat sock protein expression (HSP70, HSP27 and Hsc70) at selected time-points (n=5/group at each time). In a second protocol, we determined (n=5/group) lung weight gain (edema), pulmonary compliance, oxygenation capacity, pulmonary artery pressure (PAP) and vascular resistance (PVR), the expression of PECAM-1 (CD31) and phosphorylation status of Src-kinase and VE-cadherin in lung tissue, as well as the release in perfusate of cytokines (TNFα, IL-1β) and endothelial biomarkers (sPECAM, von Willebrand Factor -vWF-, sE-selectin and sICAM-1). Histological and immunofluorescent studies assessed perivascular edema and formation of 3-nitrotyrosine (a marker of peroxinitrite) in CD31 lung endothelium.
Results: HS induced an early (3h) and persisting expression of HSP70 and HSP27, without influencing Hsc70. Lungs from the EVLP37°C group developed massive edema, low compliance and oxygenation, elevated PAP and PVR, substantial release of TNFα, IL-1β, s-PECAM, vWF, E-selectin and s-ICAM, as well as significant Src-kinase activation, VE-cadherin phosphorylation, endothelial 3-NT formation and reduced CD31 expression. In marked contrast, all these alterations were either abrogated or significantly attenuated by HS treatment.
Conclusion: The therapeutic application of a transient heat stress during EVLP of damaged rat lungs reduces endothelial permeability, attenuates pulmonary vasoconstriction, prevents src-kinase activation and VE-cadherin phosphorylation, while reducing endothelial peroxinitrite generation and the release of cytokines and endothelial biomarkers. Collectively, these data demonstrate that therapeutic heat stress may represent a promising strategy to protect the lung endothelium from severe reperfusion injury.
Keywords: animal model; ex-vivo lung perfusion; heat shock response; heat therapy; lung ischemia-reperfusion; lung transplantation; pulmonary endothelium.
Copyright © 2024 Parapanov, Debonneville, Allouche, Lugrin, Rodriguez-Caro, Liaudet and Krueger.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
-
- Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb SB, et al. . The registry of the international society for heart and lung transplantation: thirty-second official adult lung and heart-lung transplantation report–2015; focus theme: early graft failure. J Heart Lung Transplant. (2015) 34:1264–77. doi: 10.1016/j.healun.2015.08.014 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

