Late-stage diversification of bacterial natural products through biocatalysis
- PMID: 38807651
- PMCID: PMC11130421
- DOI: 10.3389/fbioe.2024.1351583
Late-stage diversification of bacterial natural products through biocatalysis
Abstract
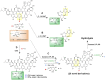
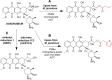
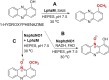
Bacterial natural products (BNPs) are very important sources of leads for drug development and chemical novelty. The possibility to perform late-stage diversification of BNPs using biocatalysis is an attractive alternative route other than total chemical synthesis or metal complexation reactions. Although biocatalysis is gaining popularity as a green chemistry methodology, a vast majority of orphan sequenced genomic data related to metabolic pathways for BNP biosynthesis and its tailoring enzymes are underexplored. In this review, we report a systematic overview of biotransformations of 21 molecules, which include derivatization by halogenation, esterification, reduction, oxidation, alkylation and nitration reactions, as well as degradation products as their sub-derivatives. These BNPs were grouped based on their biological activities into antibacterial (5), antifungal (5), anticancer (5), immunosuppressive (2) and quorum sensing modulating (4) compounds. This study summarized 73 derivatives and 16 degradation sub-derivatives originating from 12 BNPs. The highest number of biocatalytic reactions was observed for drugs that are already in clinical use: 28 reactions for the antibacterial drug vancomycin, followed by 18 reactions reported for the immunosuppressive drug rapamycin. The most common biocatalysts include oxidoreductases, transferases, lipases, isomerases and haloperoxidases. This review highlights biocatalytic routes for the late-stage diversification reactions of BNPs, which potentially help to recognize the structural optimizations of bioactive scaffolds for the generation of new biomolecules, eventually leading to drug development.
Keywords: bacterial natural products; bioactive molecules; biocatalysis; biotransformation; enzymatic diversification; late-stage modification.
Copyright © 2024 Lazic, Filipovic, Pantelic, Milovanovic, Vojnovic and Nikodinovic-Runic.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Chemoenzymatic Total Synthesis of Natural Products.Acc Chem Res. 2021 Mar 16;54(6):1374-1384. doi: 10.1021/acs.accounts.0c00810. Epub 2021 Feb 18. Acc Chem Res. 2021. PMID: 33600149 Free PMC article. Review.
-
Natural Compounds as Pharmaceuticals: The Key Role of Cytochromes P450 Reactivity.Trends Biochem Sci. 2020 Jun;45(6):511-525. doi: 10.1016/j.tibs.2020.03.004. Epub 2020 Apr 5. Trends Biochem Sci. 2020. PMID: 32413326 Review.
-
Biocatalyzed Synthesis of Glycostructures with Anti-infective Activity.Acc Chem Res. 2022 Sep 6;55(17):2409-2424. doi: 10.1021/acs.accounts.2c00136. Epub 2022 Aug 9. Acc Chem Res. 2022. PMID: 35942874 Free PMC article. Review.
-
Enzymatic Late-Stage Modifications: Better Late Than Never.Angew Chem Int Ed Engl. 2021 Jul 26;60(31):16824-16855. doi: 10.1002/anie.202014931. Epub 2021 Mar 8. Angew Chem Int Ed Engl. 2021. PMID: 33453143 Free PMC article. Review.
-
Biocatalytic Oxidation Reactions: A Chemist's Perspective.Angew Chem Int Ed Engl. 2018 Jul 20;57(30):9238-9261. doi: 10.1002/anie.201800343. Epub 2018 Jul 3. Angew Chem Int Ed Engl. 2018. PMID: 29573076 Free PMC article. Review.
Cited by
-
Current Approaches and Implications in Discovery of Novel Bioactive Products from Microbial Sources.Curr Microbiol. 2025 Apr 22;82(6):258. doi: 10.1007/s00284-025-04237-7. Curr Microbiol. 2025. PMID: 40263159 Review.
References
-
- Altreuter D. H., Dordick J. S., Clark D. S. (2002). Optimization of ion-paired lipase for non-aqueous media: acylation of doxorubicin based on surface models of fatty acid esterification. Enzyme Microb. Technol. 31, 10–19. 10.1016/s0141-0229(02)00092-3 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

