Cytokine release syndrome induced by immune checkpoint inhibitor treatment for uterine cervical cancer recurrence: A case report
- PMID: 38807673
- PMCID: PMC11130748
- DOI: 10.3892/ol.2024.14463
Cytokine release syndrome induced by immune checkpoint inhibitor treatment for uterine cervical cancer recurrence: A case report
Abstract
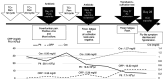
Cytokine release syndrome (CRS) is a systemic inflammatory condition caused by an excessive immune response and cytokine overproduction. CRS is a life-threatening condition that is often associated with chimeric antigen receptor T-cell therapy. Despite the increased use of immune checkpoint inhibitors (ICIs), ICI-induced CRS remains rare. The present study describes a case of CRS that occurred after the administration of ICIs for recurrent adenocarcinoma of the uterine cervix. A 49-year-old woman received paclitaxel, carboplatin and pembrolizumab for recurrent cervical adenocarcinoma. On day 27 of the third cycle, the patient was admitted with a fever and suspected pyelonephritis. The following day, hypotension, upper respiratory symptoms and myalgia of the extremities were noted, and the left ventricular ejection fraction (LVEF) was decreased to 20%. Multiorgan failure (MOF) occurred, and the patient received ventilator support and continuous hemodiafiltration. Rhabdomyolysis, pancreatitis, erythema multiforme and enteritis were observed. CRS was diagnosed based on elevated ferritin and IL-6 levels. Steroid pulse therapy was administered; however, the MOF did not improve and the anti-IL-6-receptor monoclonal antibody tocilizumab (TOC) was administered. Subsequently, the LVEF improved to 50%, and the patient was removed from the ventilator on day 4 and from the continuous hemodiafiltration unit on day 6 after TOC administration. The patient was discharged on day 21. In conclusion, considering that ICI-induced CRS is a rare but severe complication, fever and other systemic conditions following ICI administration should be monitored.
Keywords: cytokine release syndrome; immune checkpoint inhibitor; immune-related adverse events; tocilizumab; uterine cervical cancer.
Copyright: © 2024 Sekimata et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Cytokine release syndrome following COVID-19 infection during treatment with nivolumab for cancer of esophagogastric junction carcinoma: a case report and review.Int J Emerg Med. 2024 Sep 2;17(1):106. doi: 10.1186/s12245-024-00691-5. Int J Emerg Med. 2024. PMID: 39223460 Free PMC article.
-
Cytokine release syndrome following durvalumab and tremelimumab in advanced hepatocellular carcinoma: A case report with cytokine and damage-associated molecular pattern analysis.Hepatol Res. 2024 Jun 29. doi: 10.1111/hepr.14088. Online ahead of print. Hepatol Res. 2024. PMID: 38943555
-
Successful Application of Tocilizumab in a Patient With Neoadjuvant Immunochemotherapy-Induced Cytokine Release Syndrome.Cancer Rep (Hoboken). 2024 Jul;7(7):e2145. doi: 10.1002/cnr2.2145. Cancer Rep (Hoboken). 2024. PMID: 39051558 Free PMC article.
-
Cytokine Release Syndrome in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Case Series of 25 Patients and Review of the Literature.Front Immunol. 2022 Jan 28;13:807050. doi: 10.3389/fimmu.2022.807050. eCollection 2022. Front Immunol. 2022. PMID: 35154124 Free PMC article. Review.
-
Nivolumab-Induced Cytokine Release Syndrome: A Case Report and Literature Review.Am J Case Rep. 2024 Apr 16;25:e941835. doi: 10.12659/AJCR.941835. Am J Case Rep. 2024. PMID: 38625840 Free PMC article. Review.
Cited by
-
Modulation of Chronic Cytokine Dysregulation in Cervical Cancer: Potential Biomarkers and Therapeutic Targets.Cancer Manag Res. 2025 Jun 13;17:1113-1126. doi: 10.2147/CMAR.S527913. eCollection 2025. Cancer Manag Res. 2025. PMID: 40535576 Free PMC article. Review.
References
-
- Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. - DOI - PMC - PubMed
-
- Tay SH, Toh MMX, Thian YL, Vellayappan BA, Fairhurst AM, Chan YH, Aminkeng F, Bharwani LD, Huang Y, Mak A, Wong ASC. Cytokine release syndrome in cancer patients receiving immune checkpoint inhibitors: A case series of 25 patients and review of the literature. Front Immunol. 2022;13:807050. doi: 10.3389/fimmu.2022.807050. - DOI - PMC - PubMed
-
- Chennamadhavuni A, Abushahin L, Jin N, Presley CJ, Manne A. Risk factors and biomarkers for immune-related adverse events: A practical guide to identifying high-risk patients and rechallenging immune checkpoint inhibitors. Front Immunol. 2022;13:779691. doi: 10.3389/fimmu.2022.779691. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
