Physiological and transcriptomic analyses provide preliminary insights into the autotoxicity of Lilium brownii
- PMID: 38807780
- PMCID: PMC11130447
- DOI: 10.3389/fpls.2024.1330061
Physiological and transcriptomic analyses provide preliminary insights into the autotoxicity of Lilium brownii
Abstract
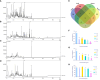
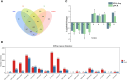
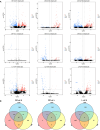
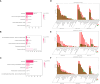
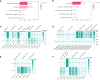
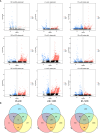
Lilium brownii F. E. Brown ex Miellez var. viridulum Baker (Longya lily) is a variety of Lilium brownii F.E. Br. ex Miellez. We used HS-SPME and GC-MS to screened the tissues of L. brownii roots, stems, bulbs, and leaves and obtained 2,4-DTBP as an autotoxic substance for subsequent analysis. 2,4-DTBP was highly autotoxic in some treatment groups. Based on changes in physiological indicators, we carried out transcriptomic analysis to investigate the mechanisms of autotoxicity of substances on L. brownii and obtained 188,505 Unigenes. GO and KEGG enrichment analyses showed that L. brownii responded differently to different concentrations and treatment times of 2,4-DTBP. We observed significant changes in genes associated with ROS, phytohormones, and MAPK signaling cascades. 2,4-DTBP affects chloroplasts, the integrity of the respiratory electron transport chain, and ribosomes, causing L. brownii autotoxicity. Our findings provide a practical genomic resource for future research on L. brownii autotoxicity and evidence for the mechanism of action of autotoxic substances.
Keywords: Lilium brownii; autotoxicity; phenolic; phytohormone; reactive oxygen species (ROS); transcriptome.
Copyright © 2024 Zhong, Guo, Su, Jiang, Wang, Shi, Li, Liao and Xue.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Identification and Characterization of Volatile Organic Compounds Based on GC-IMS Technology in Different Organs of Lilium brownii var. viridulum and After Bud-Removal and Non-Bud-Removal Treatments.Molecules. 2025 Mar 10;30(6):1238. doi: 10.3390/molecules30061238. Molecules. 2025. PMID: 40142014 Free PMC article.
-
Integrative analysis of metabolome and transcriptome provide new insights into the bitter components of Lilium lancifolium and Lilium brownii.J Pharm Biomed Anal. 2022 Jun 5;215:114778. doi: 10.1016/j.jpba.2022.114778. Epub 2022 Apr 20. J Pharm Biomed Anal. 2022. PMID: 35462288
-
Integrated transcriptomic and metabolomic analysis reveals the molecular profiles of dynamic variation in Lilium brownii var. viridulum suffering from bulb rot.Front Genet. 2024 Aug 14;15:1432997. doi: 10.3389/fgene.2024.1432997. eCollection 2024. Front Genet. 2024. PMID: 39205945 Free PMC article.
-
Lilium brownii/Baihe as Nutraceuticals: Insights into Its Composition and Therapeutic Properties.Pharmaceuticals (Basel). 2024 Sep 20;17(9):1242. doi: 10.3390/ph17091242. Pharmaceuticals (Basel). 2024. PMID: 39338404 Free PMC article. Review.
-
Anabolic metabolism of autotoxic substance coumarins in plants.PeerJ. 2023 Dec 6;11:e16508. doi: 10.7717/peerj.16508. eCollection 2023. PeerJ. 2023. PMID: 38077428 Free PMC article. Review.
References
-
- Chen P., Wang Y., Liu Q., Zhang Y., Li X., Li H., et al. . (2020. a). Phase changes of continuous cropping obstacles in strawberry (Fragaria × ananassa Duch.) production. Appl. Soil Ecol. 155, 103626. doi: 10.1016/j.apsoil.2020.103626 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

