Navigating the Link Between Non-alcoholic Fatty Liver Disease/Non-alcoholic Steatohepatitis and Cardiometabolic Syndrome
- PMID: 38807856
- PMCID: PMC11131154
- DOI: 10.15420/ecr.2023.26
Navigating the Link Between Non-alcoholic Fatty Liver Disease/Non-alcoholic Steatohepatitis and Cardiometabolic Syndrome
Abstract
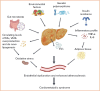
The global prevalence of non-alcoholic fatty liver disease (NAFLD) is nearly 25% and is increasing rapidly. The spectrum of liver damage in NAFLD ranges from simple steatosis to non-alcoholic steatohepatitis, characterised by the presence of lobular inflammation and hepatocyte ballooning degeneration, with or without fibrosis, which can further develop into cirrhosis and hepatocellular carcinoma. Not only is NAFLD a progressive liver disease, but numerous pieces of evidence also point to extrahepatic consequences. Accumulating evidence suggests that patients with NAFLD are also at increased risk of cardiovascular disease (CVD); in fact, CVDs are the most common cause of mortality in patients with NAFLD. Obesity, type 2 diabetes and higher levels of LDL are common risk factors in both NAFLD and CVD; however, how NAFLD affects the development and progression of CVD remains elusive. In this review, we comprehensively summarise current data on the key extrahepatic manifestations of NAFLD, emphasising the possible link between NAFLD and CVD, including the role of proprotein convertase substilisin/kenin type 9, extracellular vesicles, microbiota, and genetic factors.
Keywords: Non-alcoholic fatty liver disease (NAFLD); cardiovascular disease (CVD); extracellular vesicles; genetics; microbiota.
Copyright © The Author(s), 2024. Published by Radcliffe Group Ltd.
Conflict of interest statement
Disclosures: MRG has received grants from Andalucía se mueve con Europa, Instituto de Salud Carlos III, Gilead, Intercept and Siemens; consulting fees from Abbvie, Alpha-sigma, Allergan, AstraZeneca, Axcella, BMS, Boehringer-Ingelheim, Gilead, Inventia, Kaleido, MSD, Novo-Nordisk, Pfizer and Proscientol; honoraria from Inventia, Novo-Nordisk, Rubió, Shionogi and Sobi; travel support from Abbvie and Gilead; and participates on an advisory board for Galmed. All other authors have no conflicts of interest to declare. Funding: This work was supported by Instituto de Salud Carlos III: PFIS FI20/00201 to SGZ, IFI22/00035 to VGF, Sara Borrell CD23-00024 to AGG; Junta de Andalucía: Talento Doctores DOC_00866 to RMH and Instituto de Salud Carlos III: PMP21/00078 and PI22/01342 to MRG.
Figures
Similar articles
-
Vitamin E for people with non-alcoholic fatty liver disease.Cochrane Database Syst Rev. 2024 Oct 16;10(10):CD015033. doi: 10.1002/14651858.CD015033.pub2. Cochrane Database Syst Rev. 2024. PMID: 39412049 Free PMC article.
-
Pharmacological interventions for non-alcohol related fatty liver disease (NAFLD): an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 30;3(3):CD011640. doi: 10.1002/14651858.CD011640.pub2. Cochrane Database Syst Rev. 2017. PMID: 28358980 Free PMC article.
-
Nutritional supplementation for nonalcohol-related fatty liver disease: a network meta-analysis.Cochrane Database Syst Rev. 2021 Jul 19;7(7):CD013157. doi: 10.1002/14651858.CD013157.pub2. Cochrane Database Syst Rev. 2021. PMID: 34280304 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease.J Hepatol. 2024 May;80(5):694-701. doi: 10.1016/j.jhep.2024.01.014. Epub 2024 Jan 27. J Hepatol. 2024. PMID: 38286339
Cited by
-
Phase 3 ESSENCE Trial: Semaglutide in Metabolic Dysfunction-Associated Steatohepatitis.Gastroenterol Hepatol (N Y). 2024 Dec;20(12 Suppl 11):6-7. Gastroenterol Hepatol (N Y). 2024. PMID: 39896971 Free PMC article. No abstract available.
-
Propagermanium as a Novel Therapeutic Approach for the Treatment of Endothelial Dysfunction in Type 2 Diabetes.Int J Mol Sci. 2024 Jul 30;25(15):8328. doi: 10.3390/ijms25158328. Int J Mol Sci. 2024. PMID: 39125901 Free PMC article.
-
Recent Data From the ESSENCE Trial on Semaglutide in Metabolic Dysfunction-Associated Steatohepatitis.Gastroenterol Hepatol (N Y). 2025 Mar;21(2):133-135. Gastroenterol Hepatol (N Y). 2025. PMID: 40115605 Free PMC article. No abstract available.
-
Analysis of postprandial time trends and influencing factors of blood glucose and insulin in type 2 diabetes mellitus (T2DM) with metabolic dysfunction-associated steatotic liver disease (MASLD): a retrospective study based on propensity score matching (PSM).Acta Diabetol. 2025 Apr 1. doi: 10.1007/s00592-025-02503-5. Online ahead of print. Acta Diabetol. 2025. PMID: 40167632
-
Semaglutide 2.4 mg in Participants With Metabolic Dysfunction-Associated Steatohepatitis: Baseline Characteristics and Design of the Phase 3 ESSENCE Trial.Aliment Pharmacol Ther. 2024 Dec;60(11-12):1525-1533. doi: 10.1111/apt.18331. Epub 2024 Oct 16. Aliment Pharmacol Ther. 2024. PMID: 39412509 Free PMC article.
References
-
- European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL–EASD–EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. doi: 10.1016/j.jhep.2015.11.004. - DOI - PubMed
-
- Ballestri S, Zona S, Targher G et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936–44. doi: 10.1111/jgh.13264. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources