Genetic deletion of zinc transporter ZnT3 induces progressive cognitive deficits in mice by impairing dendritic spine plasticity and glucose metabolism
- PMID: 38807922
- PMCID: PMC11130425
- DOI: 10.3389/fnmol.2024.1375925
Genetic deletion of zinc transporter ZnT3 induces progressive cognitive deficits in mice by impairing dendritic spine plasticity and glucose metabolism
Abstract
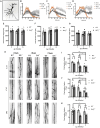
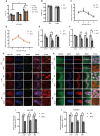
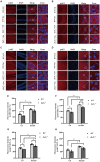
Zinc transporter 3 (ZnT3) is abundantly expressed in the brain, residing in synaptic vesicles, where it plays important roles in controlling the luminal zinc levels. In this study, we found that ZnT3 knockout in mice decreased zinc levels in the hippocampus and cortex, and was associated with progressive cognitive impairments, assessed at 2, 6, and 9-month of age. The results of Golgi-Cox staining demonstrated that ZnT3 deficiency was associated with an increase in dendritic complexity and a decrease in the density of mature dendritic spines, indicating potential synaptic plasticity deficit. Since ZnT3 deficiency was previously linked to glucose metabolism abnormalities, we tested the expression levels of genes related to insulin signaling pathway in the hippocampus and cortex. We found that the Expression of glucose transporters, GLUT3, GLUT4, and the insulin receptor in the whole tissue and synaptosome fraction of the hippocampus of the ZnT3 knockout mice were significantly reduced, as compared to wild-type controls. Expression of AKT (A serine/threonine protein kinase) and insulin-induced AKT phosphorylation was also reduced in the hippocampus of ZnT3 knockout mice. We hypothesize that the ZnT3 deficiency and reduced brain zinc levels may cause cognitive impairment by negatively affecting glycose metabolism via decreased expression of key components of insulin signaling, as well as via changes in synaptic plasticity. These finding may provide new therapeutic target for treatments of neurodegenerative disorders.
Keywords: ZnT3; dendritic plasticity; glycometabolism; spatial memory; zinc.
Copyright © 2024 Zong, Zhang, Dong, Liu, Zhang, Gao, Zhang, Ma, Gao and Gamper.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- Apelt J., Mehlhorn G., Schliebs R. (1999). Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. J. Neurosci. Res. 57, 693–705. 10.1002/(SICI)1097-4547(19990901)57:5<693::AID-JNR11>3.0.CO;2-X - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

