Arrhythmia monitoring and outcome after myocardial infarction (BIO|GUARD-MI): a randomized trial
- PMID: 38807948
- PMCID: PMC11132184
- DOI: 10.3389/fcvm.2024.1300074
Arrhythmia monitoring and outcome after myocardial infarction (BIO|GUARD-MI): a randomized trial
Abstract
Objectives: Cardiac arrhythmias predict poor outcome after myocardial infarction (MI). We studied if arrhythmia monitoring with an insertable cardiac monitor (ICM) can improve treatment and outcome.
Design: BIO|GUARD-MI was a randomized, international open-label study with blinded outcome assessment.
Setting: Tertiary care facilities monitored the arrhythmias, while the follow-up remained with primary care physicians.
Participants: Patients after ST-elevation (STEMI) or non-ST-elevation MI with an ejection fraction >35% and a CHA2DS2-VASc score ≥4 (men) or ≥5 (women).
Interventions: Patients were randomly assigned to receive or not receive an ICM in addition to standard post-MI treatment. Device-detected arrhythmias triggered immediate guideline recommended therapy changes via remote monitoring.
Main outcome measures: MACE, defined as a composite of cardiovascular death or acute unscheduled hospitalization for cardiovascular causes.
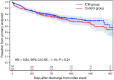
Results: 790 patients (mean age 71 years, 72% male, 51% non-STEMI) of planned 1,400 pts were enrolled and followed for a median of 31.6 months. At 2 years, 39.4% of the device group and 6.7% of the control group had their therapy adapted for an arrhythmia [hazard ratio (HR) = 5.9, P < 0.0001]. Most frequent arrhythmias were atrial fibrillation, pauses and bradycardia. The use of an ICM did not improve outcome in the entire cohort (HR = 0.84, 95%-CI: 0.65-1.10; P = 0.21). In secondary analysis, a statistically significant interaction of the type of infarction suggests a benefit in the pre-specified non-STEMI subgroup. Risk factor analysis indicates that this may be connected to the higher incidence of MACE in patients with non-STEMI.
Conclusions: The burden of asymptomatic but actionable arrhythmias is large in post-infarction patients. However, arrhythmia monitoring with an ICM did not improve outcome in the entire cohort. Post-hoc analysis suggests that it may be beneficial in non-STEMI patients or other high-risk subgroups.
Clinical trial registration: [https://www.clinicaltrials.gov/ct2/show/NCT02341534], NCT02341534.
Keywords: cardiac arrhythmia; implantable cardiac monitor; myocardial infarction; randomized controlled trial; telemedicine.
© 2024 Jøns, Bloch Thomsen, Riahi, Smilde, Bach, Jacobsen, Táborský, Faluközy, Wiemer, Christensen, Kónyi, Schelfaut, Bulava, Grabowski, Merkely, Nuyens, Mahajan, Nagel, Tilz, Malczynski, Steinwender, Brachmann, Serota, Schrader, Behrens and Søgaard.
Conflict of interest statement
UB has received lecture fees from Boehringer, Astra Zeneca and Bayer, and travel costs from Boehringer, Astra Zeneca, Bayer, and Bristol Myers Squibb. MG reports consulting and lecture fees from Abbott Medical, Biotronik, Boston Sc. and Medtronic. BM reports grant or contract payments from Medtronic and Boston Scientific and lecture fees from Biotronik and Abbott Medical. RM reports grant or contract payments from Abbott Medical, Medtronic, Bayer and lecture fees from Bayer. RT reports consulting fees from Abbott and Boston Sc. and honoraria for lectures from Abbott Medical, Biotronik and Boston Sc. JS is an employee of Biotronik. SB reports lecture fees and travel costs from Biotronik in the context of this study, and lecture fees from Astra Zeneca, Bayer, Berlin Chemie, Boehringer, Bristol Myers Squibb, and Novartis, and DSMB or advisory board membership fees from Astra Zeneca, Bayer, Berlin Chemie, Boehringer, Bristol Myers Squibb, and Novartis. The authors declare that this study received funding from Biotronik SE & Co. KG. The funder supported study design, data collection, analysis, interpretation of data and the writing of this article.
Figures



References
-
- Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH. The CONNECT (clinical evaluation of remote notification to reduce time to clinical decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. (2011) 57:1181–9. 10.1016/j.jacc.2010.12.012 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

