Multiple treatment interruptions and protecting HIV-specific CD4 T cells enable durable CD8 T cell response and viral control
- PMID: 38808136
- PMCID: PMC11130509
- DOI: 10.3389/fmed.2024.1342476
Multiple treatment interruptions and protecting HIV-specific CD4 T cells enable durable CD8 T cell response and viral control
Abstract
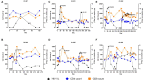
Human Immunodeficiency Virus (HIV) remains a global health challenge, and novel approaches to improve HIV control are significantly important. The cell and gene therapy product AGT103-T was previously evaluated (NCT04561258) for safety, immunogenicity, and persistence in seven patients for up to 180 days post infusion. In this study, we sought to investigate the impact of AGT103-T treatment upon analytical treatment interruptions (ATIs). Six patients previously infused with AGT103-T were enrolled into an ATI study (NCT05540964), wherein they suspended their antiretroviral therapy (ART) until their viral load reached 100,000 copies/mL in two successive visits, or their CD4 count was reduced to below 300 cells/μL. During the ATI, all patients experienced viral rebound followed by a notable expansion in HIV specific immune responses. The participants demonstrated up to a five-fold increase in total CD8 counts over baseline approximately 1-2 weeks followed by the peak viremia. This coincided with a rise in HIV-specific CD8 T cells, which was attributed to the increase in antigen availability and memory recall. Thus, the protocol was amended to include a second ATI with the first ATI serving as an "auto-vaccination." Four patients participated in a second ATI. During the second ATI, the Gag-specific CD8 T cells were either maintained or rose in response to viral rebound and the peak viremia was substantially decreased. The patients reached a viral set point ranging from 7,000 copies/mL to 25,000 copies/mL. Upon resuming ART, all participants achieved viral control more rapidly than during the first ATI, with CD4 counts remaining within 10% of baseline measurements and without any serious adverse events or evidence of drug resistance. In summary, the rise in CD8 counts and the viral suppression observed in 100% of the study participants are novel observations demonstrating that AGT103-T gene therapy when combined with multiple ATIs, is a safe and effective approach for achieving viral control, with viral setpoints consistently below 25,000 copies/mL and relatively stable CD4 T cell counts. We conclude that HIV cure-oriented cell and gene therapy trials should include ATI and may benefit from designs that include multiple ATIs when induction of CD8 T cells is required to establish viral control.
Keywords: AGT103-T; CCR5; CD4 /CD8 lymphocytes + +; Human Immunodeficiency Virus (HIV); cell and gene therapy (CGT); immunotherapy.
Copyright © 2024 Jain, Canepa, Liou, Fledderman, Chapoval, Xiao, Mukherjee, Balogun, Huaman-Vergara, Galvin, Kumar, Bordon, Conant and Boyle.
Conflict of interest statement
Authors AJ, GC, M-LL, EF, AC, LX, IM, BB, HH-V, JG, MC, and JeB are shareholders and current employees of American Gene Technologies International, Inc. Authors PK and JoB received funding for the costs of the clinical trial from American Gene Technologies International, Inc.
Figures


References
-
- Wooldridge L, Sewell A. HIV strategically targets HIV-specific T cells. Trends Immunol. (2002) 23:474. doi: 10.1016/S1471-4906(02)02312-8 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

