Conceptualisation and Role of Market Access in Pharmaceutical Industry: A Scoping Review
- PMID: 38808312
- PMCID: PMC11130876
- DOI: 10.3390/jmahp12020007
Conceptualisation and Role of Market Access in Pharmaceutical Industry: A Scoping Review
Abstract
Background: Understanding the concept and dynamic process of the evolution of professional identity and roles of market access (MA) in the pharmaceutical industry (pharma) is critical to personal, interpersonal, and professional levels of development and impact.
Objective: The aim was to carry out a scoping review of the conceptualisation of MA within pharma.
Data sources: BioMed Central, WorldCat.org, and Directory of Open Access Journals were searched from 2003 to 2023.
Study selection: All articles on concepts or definitions and other surrogate terms on MA in pharma were selected.
Data extraction: Keywords generated from an initial cursory literature search on MA in pharma were used in conjunction with AND/OR as search terms. Using the data charting method, key findings were mapped and summarised descriptively. inductive analysis was performed, allowing codes/themes that are relevant to the concept to emerge.
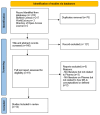
Data synthesis: Arskey and O'Malley's six-stage framework and the PRISMA extension for scoping reviews extension checklist were used as the review and reporting templates. The databases search yielded 222 results. Following title and abstract screening, a total of 146 papers were screened, and 127 of them were excluded. Full-text review was conducted for 19 papers that were deemed by two reviewers to meet the eligibility criteria. One of the authors arbitrated on disputed papers for inclusion. Only 14 of the included papers were found to meet the criteria for the final analysis. Five conceptual dimensions of MA in pharma were identified as "right products", "right patient", "right price", "right point" (time), and "right place" (setting).
Conclusions: Market access in pharma is a process that commences with the development and availability of the right products that are proven to be efficacious and disease/condition-specific (including medications, medical devices, and vaccines); specifically produced for the right patients or end users who will maximise best clinical outcomes and economic value; delivered at the right point in a timely, sustained, and efficient manner, given at the right price (commercially viable or reimbursed price that represents good value); and conducted within the economic, policy, societal, and technological contexts, with the overarching goal of achieving the best patient outcomes and ensuring product profitability.
Keywords: market access; pharmaceutical industry; professional; scoping review.
© 2024 by the authors.
Conflict of interest statement
Conflicts of InterestThe authors declare no conflicts of interest.
Figures
References
-
- World Trade Organisation What is the World Trade Organization? Simply Put Is It a Bird, Is It a Plane? [(accessed on 17 November 2022)]. Available online: https://www.wto.org/english/thewto_e/whatis_e/tif_e/fact1_e.htm.
-
- World Trade Organisation Market Access for Goods. [(accessed on 6 October 2022)]. Available online: https://www.wto.org/english/tratop_e/markacc_e/markacc_e.htm#:~:text=Mar....
-
- Kenton W. Strength, Weakness, Opportunity, and Threat (SWOT) Analysis. 2021. [(accessed on 24 February 2023)]. Available online: https://www.investopedia.com/terms/s/swot.asp.
-
- PMLiVE The True Meaning of Market Access? 2012. [(accessed on 17 July 2023)]. Available online: https://www.pmlive.com/pharma_intelligence/the_true_meaning_of_market_ac....
Publication types
LinkOut - more resources
Full Text Sources