Aging related decreases in NM myosin expression and contractility in a resistance vessel
- PMID: 38808359
- PMCID: PMC11130448
- DOI: 10.3389/fphys.2024.1411420
Aging related decreases in NM myosin expression and contractility in a resistance vessel
Abstract
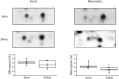
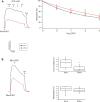
Introduction: Vasodilatation in response to NO is a fundamental response of the vasculature, and during aging, the vasculature is characterized by an increase in stiffness and decrease in sensitivity to NO mediated vasodilatation. Vascular tone is regulated by the activation of smooth muscle and nonmuscle (NM) myosin, which are regulated by the activities of myosin light chain kinase (MLCK) and MLC phosphatase. MLC phosphatase is a trimeric enzyme with a catalytic subunit, myosin targeting subunit (MYPT1) and 20 kDa subunit of unknown function. Alternative mRNA splicing produces LZ+/LZ- MYPT1 isoforms and the relative expression of LZ+/LZ- MYPT1 determines the sensitivity to NO mediated vasodilatation. This study tested the hypothesis that aging is associated with changes in LZ+ MYPT1 and NM myosin expression, which alter vascular reactivity. Methods: We determined MYPT1 and NM myosin expression, force and the sensitivity of both endothelial dependent and endothelial independent relaxation in tertiary mesenteric arteries of young (6mo) and elderly (24mo) Fischer344 rats. Results: The data demonstrate that aging is associated with a decrease in both the expression of NM myosin and force, but LZ+ MYPT expression and the sensitivity to both endothelial dependent and independent vasodilatation did not change. Further, smooth muscle cell hypertrophy increases the thickness of the medial layer of smooth muscle with aging. Discussion: The reduction of NM myosin may represent an aging associated compensatory mechanism to normalize the stiffness of resistance vessels in response to the increase in media thickness observed during aging.
Keywords: MYPT1; NM myosin; NO; endothelial independent and dependent vasodilatation; vascular reactivity.
Copyright © 2024 Han, Bandi, Fogarty, Sieck and Brozovich.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



Similar articles
-
Vascular reactivity in heart failure: role of myosin light chain phosphatase.Circ Res. 2004 Sep 17;95(6):612-8. doi: 10.1161/01.RES.0000142736.39359.58. Epub 2004 Aug 19. Circ Res. 2004. PMID: 15321927
-
Differential phosphorylation of LZ+/LZ- MYPT1 isoforms regulates MLC phosphatase activity.Arch Biochem Biophys. 2014 Nov 15;562:37-42. doi: 10.1016/j.abb.2014.08.011. Epub 2014 Aug 26. Arch Biochem Biophys. 2014. PMID: 25168281
-
MYPT1 protein isoforms are differentially phosphorylated by protein kinase G.J Biol Chem. 2011 Oct 28;286(43):37274-9. doi: 10.1074/jbc.M111.282905. Epub 2011 Sep 2. J Biol Chem. 2011. PMID: 21890627 Free PMC article.
-
The potential role of MLC phosphatase and MAPK signalling in the pathogenesis of vascular dysfunction in heart failure.J Cell Mol Med. 2008 Dec;12(6A):2158-64. doi: 10.1111/j.1582-4934.2008.00536.x. J Cell Mol Med. 2008. PMID: 19120700 Free PMC article. Review.
-
Regulation of Smooth Muscle Myosin Light Chain Phosphatase by Multisite Phosphorylation of the Myosin Targeting Subunit, MYPT1.Cardiovasc Hematol Disord Drug Targets. 2018;18(1):4-13. doi: 10.2174/1871529X18666180326120638. Cardiovasc Hematol Disord Drug Targets. 2018. PMID: 29577868 Review.
Cited by
-
Diaphragm muscle: a pump that can not fail.Physiol Rev. 2025 Oct 1;105(4):2589-2656. doi: 10.1152/physrev.00043.2024. Epub 2025 Jul 11. Physiol Rev. 2025. PMID: 40643074 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

