Mechanism of protective actions of sparsentan in the kidney: lessons from studies in models of chronic kidney disease
- PMID: 38808486
- PMCID: PMC11139641
- DOI: 10.1042/CS20240249
Mechanism of protective actions of sparsentan in the kidney: lessons from studies in models of chronic kidney disease
Abstract
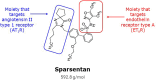
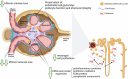
Simultaneous inhibition of angiotensin II AT1 and endothelin ETA receptors has emerged as a promising approach for treatment of chronic progressive kidney disease. This therapeutic approach has been advanced by the introduction of sparsentan, the first dual AT1 and ETA receptor antagonist. Sparsentan is a single molecule with high affinity for both receptors. It is US Food and Drug Administration approved for immunoglobulin A nephropathy (IgAN) and is currently being developed as a treatment for rare kidney diseases, such as focal segmental glomerulosclerosis. Clinical studies have demonstrated the efficacy and safety of sparsentan in these conditions. In parallel with clinical development, studies have been conducted to elucidate the mechanisms of action of sparsentan and its position in the context of published evidence characterizing the nephroprotective effects of dual ETA and AT1 receptor inhibition. This review summarizes this evidence, documenting beneficial anti-inflammatory, antifibrotic, and hemodynamic actions of sparsentan in the kidney and protective actions in glomerular endothelial cells, mesangial cells, the tubulointerstitium, and podocytes, thus providing the rationale for the use of sparsentan as therapy for focal segmental glomerulosclerosis and IgAN and suggesting potential benefits in other renal diseases, such as Alport syndrome.
Keywords: Angiotensin II; Endothelin Type A receptor; Endothelin-1; FSGS; IgA nephropathy; Sparsentan.
© 2024 The Author(s).
Conflict of interest statement
D.E.K is a consultant for Chinook Therapeutics and Travere Therapeutics, Inc. P.W.B., C.J., B.H., and R.K. are employees and shareholders of Travere Therapeutics, Inc.
Figures

References
-
- Murugesan N., Gu Z., Fadnis L., Tellew J.E., Baska R.A., Yang Y.et al. (2005) Dual angiotensin II and endothelin A receptor antagonists: synthesis of 2'-substituted N-3-isoxazolyl biphenylsulfonamides with improved potency and pharmacokinetics. J. Med. Chem. 48, 171–179 10.1021/jm049548x - DOI - PubMed
-
- Rovin B.H., Barratt J., Heerspink H.J.L., Alpers C.E., Bieler S., Chae D.W.et al. (2023) Efficacy and safety of sparsentan versus irbesartan in patients with IgA nephropathy (PROTECT): 2-year results from a randomised, active-controlled, phase 3 trial. Lancet 402, 2077–2090 10.1016/S0140-6736(23)02302-4 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

