Cardiovascular Magnetic Resonance Radiomics to Identify Components of the Extracellular Matrix in Dilated Cardiomyopathy
- PMID: 38808522
- PMCID: PMC11216881
- DOI: 10.1161/CIRCULATIONAHA.123.067107
Cardiovascular Magnetic Resonance Radiomics to Identify Components of the Extracellular Matrix in Dilated Cardiomyopathy
Abstract
Background: Current cardiovascular magnetic resonance sequences cannot discriminate between different myocardial extracellular space (ECSs), including collagen, noncollagen, and inflammation. We sought to investigate whether cardiovascular magnetic resonance radiomics analysis can distinguish between noncollagen and inflammation from collagen in dilated cardiomyopathy.
Methods: We identified data from 132 patients with dilated cardiomyopathy scheduled for an invasive septal biopsy who underwent cardiovascular magnetic resonance at 3 T. Cardiovascular magnetic resonance imaging protocol included native and postcontrast T1 mapping and late gadolinium enhancement (LGE). Radiomic features were computed from the midseptal myocardium, near the biopsy region, on native T1, extracellular volume (ECV) map, and LGE images. Principal component analysis was used to reduce the number of radiomic features to 5 principal radiomics. Moreover, a correlation analysis was conducted to identify radiomic features exhibiting a strong correlation (r>0.9) with the 5 principal radiomics. Biopsy samples were used to quantify ECS, myocardial fibrosis, and inflammation.
Results: Four histopathological phenotypes were identified: low collagen (n=20), noncollagenous ECS expansion (n=49), mild to moderate collagenous ECS expansion (n=42), and severe collagenous ECS expansion (n=21). Noncollagenous expansion was associated with the highest risk of myocardial inflammation (65%). Although native T1 and ECV provided high diagnostic performance in differentiating severe fibrosis (C statistic, 0.90 and 0.90, respectively), their performance in differentiating between noncollagen and mild to moderate collagenous expansion decreased (C statistic: 0.59 and 0.55, respectively). Integration of ECV principal radiomics provided better discrimination and reclassification between noncollagen and mild to moderate collagen (C statistic, 0.79; net reclassification index, 0.83 [95% CI, 0.45-1.22]; P<0.001). There was a similar trend in the addition of native T1 principal radiomics (C statistic, 0.75; net reclassification index, 0.93 [95% CI, 0.56-1.29]; P<0.001) and LGE principal radiomics (C statistic, 0.74; net reclassification index, 0.59 [95% CI, 0.19-0.98]; P=0.004). Five radiomic features per sequence were identified with correlation analysis. They showed a similar improvement in performance for differentiating between noncollagen and mild to moderate collagen (native T1, ECV, LGE C statistic, 0.75, 0.77, and 0.71, respectively). These improvements remained significant when confined to a single radiomic feature (native T1, ECV, LGE C statistic, 0.71, 0.70, and 0.64, respectively).
Conclusions: Radiomic features extracted from native T1, ECV, and LGE provide incremental information that improves our capability to discriminate noncollagenous expansion from mild to moderate collagen and could be useful for detecting subtle chronic inflammation in patients with dilated cardiomyopathy.
Keywords: cardiomyopathy, dilated; histology; magnetic resonance imaging; radiomics.
Conflict of interest statement
Figures


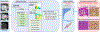
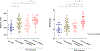
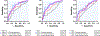

References
-
- Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. 1994;331:1564–1575. - PubMed
-
- Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation. 1996;93:841–842. - PubMed
-
- Kühl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, Poller W, Kandolf R, Schultheiss HP. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111:887–893. - PubMed
-
- Ohta-Ogo K, Sugano Y, Ogata S, Nakayama T, Komori T, Eguchi K, Dohi K, Yokokawa T, Kanamori H, Nishimura S, Nakamura K, Ikeda Y, Nishimura K, Takemura G, Anzai T, Hiroe M, Hatakeyama K, Ishibashi-Ueda H, Imanaka-Yoshida K. Myocardial T-lymphocytes as a prognostic risk-stratifying marker of dilated cardiomyopathy - results of the multicenter registry to investigate inflammatory cell infiltration in dilated cardiomyopathy in tissues of endomyocardial biopsy (INDICATE Study). Circ J 2022;86:1092–1101. - PubMed
-
- Manolio TA, Baughman KL, Rodeheffer R, Pearson TA, Bristow JD, Michels VV, Abelmann WH, Harlan WR. Prevalence and etiology of idiopathic dilated cardiomyopathy (summary of a National Heart, Lung, and Blood Institute Workshop. Am J Cardiol. 1992;69:1458–1466. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

