Mouse KL2 is a unique MTSE involved in chromosome-based spindle organization and regulated by multiple kinases during female meiosis
- PMID: 38808565
- PMCID: PMC11461529
- DOI: 10.7555/JBR.37.20230290
Mouse KL2 is a unique MTSE involved in chromosome-based spindle organization and regulated by multiple kinases during female meiosis
Abstract
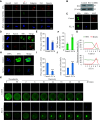
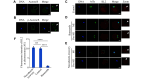
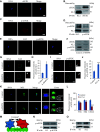
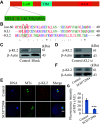
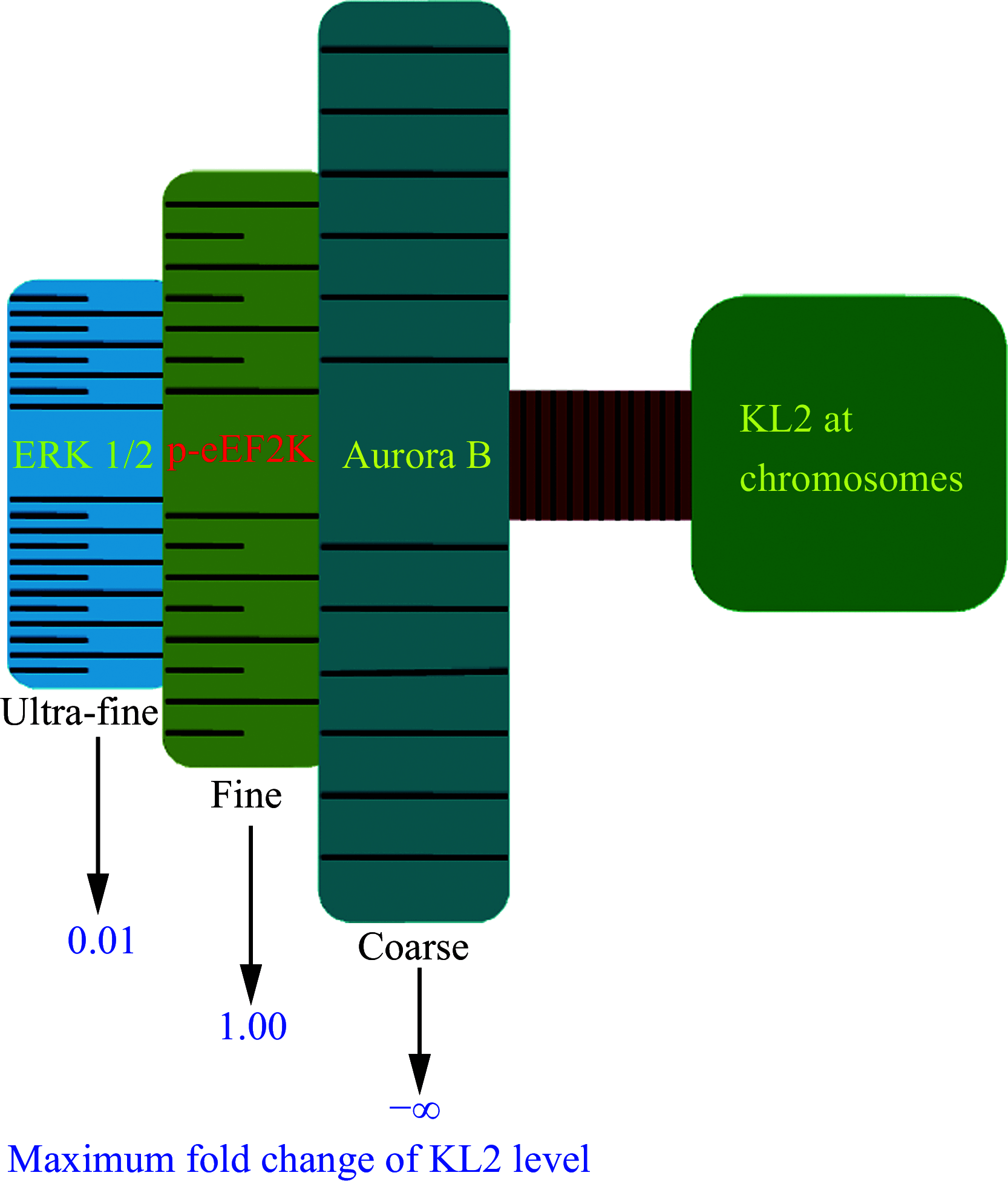
Microtubule-severing enzymes (MTSEs) play important roles in mitosis and meiosis of the primitive organisms. However, their roles in mammalian female meiosis, which accounts for over 80% of gamete-originated human reproductive diseases, remain unexplored. In the current study, we reported that katanin-like 2 (KL2) was the only MTSE concentrating at chromosomes. Furthermore, the knockdown of KL2 significantly reduced the chromosome-based increase in the microtubule (MT) polymer, increased aberrant kinetochore-MT (K-MT) attachment, delayed meiosis, and severely affected normal fertility. We demonstrated that the inhibition of aurora B, a key kinase for correcting aberrant K-MT attachment, significantly eliminated KL2 expression from chromosomes. Additionally, KL2 interacted with phosphorylated eukaryotic elongation factor-2 kinase, and they competed for chromosome binding. Phosphorylated KL2 was also localized at spindle poles, with its phosphorylation regulated by extracellular signal-regulated kinase 1/2. In summary, the current study reveals a novel function of MTSEs in mammalian female meiosis and demonstrates that multiple kinases coordinate to regulate the levels of KL2 at chromosomes.
Keywords: KL2; MTSE; female meiosis; kinase; mouse.
Conflict of interest statement
The authors reported no conflict of interests.
Figures

References
-
- Walczak CE, Heald R Mechanisms of mitotic spindle assembly and function. https://www.sciencedirect.com/science/article/abs/pii/S0074769607650037. Int Rev Cytol. 2008;265:111–158. - PubMed
LinkOut - more resources
Full Text Sources

