PHF2 regulates genome topology and DNA replication in neural stem cells via cohesin
- PMID: 38808662
- PMCID: PMC11229317
- DOI: 10.1093/nar/gkae457
PHF2 regulates genome topology and DNA replication in neural stem cells via cohesin
Abstract
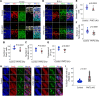
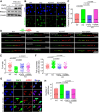
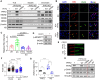
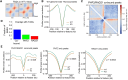
Cohesin plays a crucial role in the organization of topologically-associated domains (TADs), which influence gene expression and DNA replication timing. Whether epigenetic regulators may affect TADs via cohesin to mediate DNA replication remains elusive. Here, we discover that the histone demethylase PHF2 associates with RAD21, a core subunit of cohesin, to regulate DNA replication in mouse neural stem cells (NSC). PHF2 loss impairs DNA replication due to the activation of dormant replication origins in NSC. Notably, the PHF2/RAD21 co-bound genomic regions are characterized by CTCF enrichment and epigenomic features that resemble efficient, active replication origins, and can act as boundaries to separate adjacent domains. Accordingly, PHF2 loss weakens TADs and chromatin loops at the co-bound loci due to reduced RAD21 occupancy. The observed topological and DNA replication defects in PHF2 KO NSC support a cohesin-dependent mechanism. Furthermore, we demonstrate that the PHF2/RAD21 complex exerts little effect on gene regulation, and that PHF2's histone-demethylase activity is dispensable for normal DNA replication and proliferation of NSC. We propose that PHF2 may serve as a topological accessory to cohesin for cohesin localization to TADs and chromatin loops, where cohesin represses dormant replication origins directly or indirectly, to sustain DNA replication in NSC.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- Nasmyth K., Haering C.H.. Cohesin: its roles and mechanisms. Annu. Rev. Genet. 2009; 43:525–558. - PubMed
-
- Waldman T. Emerging themes in cohesin cancer biology. Nat. Rev. Cancer. 2020; 20:504–515. - PubMed
-
- Peters J.M., Tedeschi A., Schmitz J.. The cohesin complex and its roles in chromosome biology. Genes Dev. 2008; 22:3089–3114. - PubMed
-
- Wutz G., Varnai C., Nagasaka K., Cisneros D.A., Stocsits R.R., Tang W., Schoenfelder S., Jessberger G., Muhar M., Hossain M.J.et al. .. Topologically associating domains and chromatin loops depend on cohesin and are regulated by CTCF, WAPL, and PDS5 proteins. EMBO J. 2017; 36:3573–3599. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

