Trimethylamine-N-oxide promotes osteoclast differentiation and oxidative stress by activating NF-κB pathway
- PMID: 38809508
- PMCID: PMC11164488
- DOI: 10.18632/aging.205869
Trimethylamine-N-oxide promotes osteoclast differentiation and oxidative stress by activating NF-κB pathway
Abstract
Background: Senile osteoporosis may be caused by an imbalance in intestinal flora and oxidative stress. Trimethylamine-N-oxide (TMAO), a metabolite of dietary choline dependent on gut microbes, has been found to be significantly increased in osteoporosis. However, the role of TMAO in bone loss during osteoporosis remains poorly understood. In this study, we examined the impact of TMAO on osteoclast differentiation and bone resorption in an in vitro setting.
Methods: Osteoclast differentiation was induced by incubating RAW 264.7 cells in the presence of Receptor Activator for Nuclear Factor-κB Ligand (RANKL) and macrophage-stimulating factor (M-CSF). Flow cytometry, TRAP staining assay, CCK-8, and ELISA were employed to investigate the impact of TMAO on osteoclast differentiation and bone resorption activity in vitro. For mechanistic exploration, RT-PCR and Western blotting were utilized to assess the activation of the NF-κB pathway. Additionally, protein levels of secreted cytokines and growth factors were determined using suspension array technology.
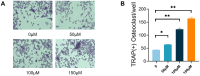
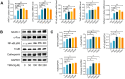
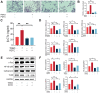
Results: Our findings demonstrate that TMAO enhances RANKL and M-CSF-induced osteoclast formation and bone resorption in a dose-dependent manner. Mechanistically, TMAO triggers the upregulation of the NF-κB pathway and osteoclast-related genes (NFATc1, c-Fos, NF-κB p65, Traf6, and Cathepsin K). Furthermore, TMAO markedly elevated the levels of oxidative stress and inflammatory factors.
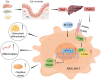
Conclusions: In conclusion, TMAO enhances RANKL and M-CSF-induced osteoclast differentiation and inflammation in RAW 264.7 cells by activating the NF-κB signaling pathway. These findings offer a new rationale for further academic and clinical research on osteoporosis treatment.
Keywords: NF-κB; inflammation; osteoclast; reactive oxygen species; trimethylamine-N-oxide.
Conflict of interest statement
Figures



References
-
- Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999; 402:304–9. 10.1038/46303 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

