Elevated PINK1/Parkin-Dependent Mitophagy and Boosted Mitochondrial Function Mediate Protection of HepG2 Cells from Excess Palmitic Acid by Hesperetin
- PMID: 38809522
- PMCID: PMC11181321
- DOI: 10.1021/acs.jafc.3c09132
Elevated PINK1/Parkin-Dependent Mitophagy and Boosted Mitochondrial Function Mediate Protection of HepG2 Cells from Excess Palmitic Acid by Hesperetin
Abstract
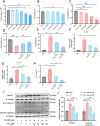
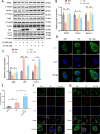
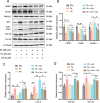
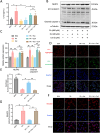
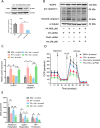
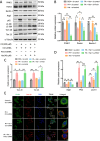
Deregulation of mitochondrial functions in hepatocytes contributes to many liver diseases, such as nonalcoholic fatty liver disease (NAFLD). Lately, it was referred to as MAFLD (metabolism-associated fatty liver disease). Hesperetin (Hst), a bioactive flavonoid constituent of citrus fruit, has been proven to attenuate NAFLD. However, a potential connection between its preventive activities and the modulation of mitochondrial functions remains unclear. Here, our results showed that Hst alleviates palmitic acid (PA)-triggered NLRP3 inflammasome activation and cell death by inhibition of mitochondrial impairment in HepG2 cells. Hst reinstates fatty acid oxidation (FAO) rates measured by seahorse extracellular flux analyzer and intracellular acetyl-CoA levels as well as intracellular tricarboxylic acid cycle metabolites levels including NADH and FADH2 reduced by PA exposure. In addition, Hst protects HepG2 cells against PA-induced abnormal energetic profile, ATP generation reduction, overproduction of mitochondrial reactive oxygen species, and collapsed mitochondrial membrane potential. Furthermore, Hst improves the protein expression involved in PINK1/Parkin-mediated mitophagy. Our results demonstrate that it restores PA-impaired mitochondrial function and sustains cellular homeostasis due to the elevation of PINK1/Parkin-mediated mitophagy and the subsequent disposal of dysfunctional mitochondria. These results provide therapeutic potential for Hst utilization as an effective intervention against fatty liver disease.
Keywords: PINK1/Parkin-mediated mitophagy degradation; TCA cycle and fatty acid oxidation; hesperetin; metabolomics; mitochondrial dysfunction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

