Preictal dysfunctions of inhibitory interneurons paradoxically lead to their rebound hyperactivity and to low-voltage-fast onset seizures in Dravet syndrome
- PMID: 38809712
- PMCID: PMC11161744
- DOI: 10.1073/pnas.2316364121
Preictal dysfunctions of inhibitory interneurons paradoxically lead to their rebound hyperactivity and to low-voltage-fast onset seizures in Dravet syndrome
Abstract
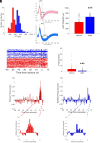
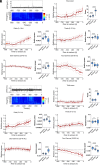
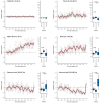
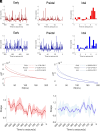
Epilepsies have numerous specific mechanisms. The understanding of neural dynamics leading to seizures is important for disclosing pathological mechanisms and developing therapeutic approaches. We investigated electrographic activities and neural dynamics leading to convulsive seizures in patients and mouse models of Dravet syndrome (DS), a developmental and epileptic encephalopathy in which hypoexcitability of GABAergic neurons is considered to be the main dysfunction. We analyzed EEGs from DS patients carrying a SCN1A pathogenic variant, as well as epidural electrocorticograms, hippocampal local field potentials, and hippocampal single-unit neuronal activities in Scn1a+/- and Scn1aRH/+ DS mice. Strikingly, most seizures had low-voltage-fast onset in both patients and mice, which is thought to be generated by hyperactivity of GABAergic interneurons, the opposite of the main pathological mechanism of DS. Analyzing single-unit recordings, we observed that temporal disorganization of the firing of putative interneurons in the period immediately before the seizure (preictal) precedes the increase of their activity at seizure onset, together with the entire neuronal network. Moreover, we found early signatures of the preictal period in the spectral features of hippocampal and cortical field potential of Scn1a mice and of patients' EEG, which are consistent with the dysfunctions that we observed in single neurons and that allowed seizure prediction. Therefore, the perturbed preictal activity of interneurons leads to their hyperactivity at the onset of generalized seizures, which have low-voltage-fast features that are similar to those observed in other epilepsies and are triggered by hyperactivity of GABAergic neurons. Preictal spectral features may be used as predictive seizure biomarkers.
Keywords: GABA; epilepsy; excitability; seizure prediction; sodium channels.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Similar articles
-
A Transient Developmental Window of Fast-Spiking Interneuron Dysfunction in a Mouse Model of Dravet Syndrome.J Neurosci. 2018 Sep 5;38(36):7912-7927. doi: 10.1523/JNEUROSCI.0193-18.2018. Epub 2018 Aug 13. J Neurosci. 2018. PMID: 30104343 Free PMC article.
-
Impairment of Sharp-Wave Ripples in a Murine Model of Dravet Syndrome.J Neurosci. 2019 Nov 13;39(46):9251-9260. doi: 10.1523/JNEUROSCI.0890-19.2019. Epub 2019 Sep 19. J Neurosci. 2019. PMID: 31537705 Free PMC article.
-
Interneuron Desynchronization Precedes Seizures in a Mouse Model of Dravet Syndrome.J Neurosci. 2020 Mar 25;40(13):2764-2775. doi: 10.1523/JNEUROSCI.2370-19.2020. Epub 2020 Feb 26. J Neurosci. 2020. PMID: 32102923 Free PMC article.
-
Dravet syndrome: the main issues.Eur J Paediatr Neurol. 2012 Sep;16 Suppl 1:S1-4. doi: 10.1016/j.ejpn.2012.04.006. Epub 2012 Jun 15. Eur J Paediatr Neurol. 2012. PMID: 22705271 Review.
-
Dravet Syndrome: Diagnosis and Long-Term Course.Can J Neurol Sci. 2016 Jun;43 Suppl 3:S3-8. doi: 10.1017/cjn.2016.243. Can J Neurol Sci. 2016. PMID: 27264139 Review.
Cited by
-
Directionality of neural activity in and out of the seizure onset zone in focal epilepsy.Netw Neurosci. 2025 Jun 30;9(2):798-823. doi: 10.1162/netn_a_00454. eCollection 2025. Netw Neurosci. 2025. PMID: 40612715 Free PMC article.
References
-
- Dravet C., Dravet syndrome history. Dev. Med. Child. Neurol. 53, 1–6 (2011). - PubMed
-
- Guerrini R., Cross H., Nabbout R., Dalla Bernardina B., Dravet syndrome and other sodium channel-related encephalopathies. Epilepsia 60, S1 (2019). - PubMed
-
- Zuberi S. M., et al. , ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 63, 1349–1397 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

