Recombinant Alpha-1 Antitrypsin-Fc Fusion Protein INBRX-101 in Adults With Alpha-1 Antitrypsin Deficiency: A Phase 1 Study
- PMID: 38809792
- PMCID: PMC11216232
- DOI: 10.15326/jcopdf.2023.0469
Recombinant Alpha-1 Antitrypsin-Fc Fusion Protein INBRX-101 in Adults With Alpha-1 Antitrypsin Deficiency: A Phase 1 Study
Abstract
Background: Alpha-1 antitrypsin deficiency (AATD) is characterized by low alpha-1 antitrypsin (AAT) levels, predisposing individuals to lung disease. The standard of care, plasma-derived AAT (pdAAT), is delivered as weekly infusions to maintain serum AAT concentrations ≥11µM (≈50% of those in healthy individuals). INBRX-101, a recombinant human AAT-Fc fusion protein, was designed to have a longer half-life and achieve higher AAT levels than pdAAT.
Methods: In this phase 1 dose-escalation study (N=31), adults with AATD received 1 dose (part 1) or 3 doses (part 2) of 10 (part 1), 40, 80, or 120mg/kg INBRX-101 every 3 weeks (Q3W) via intravenous infusion. The primary endpoint was safety and tolerability. Secondary endpoints were pharmacokinetics (PK), pharmacodynamics (PD), and immunogenicity of INBRX-101.
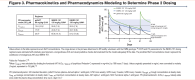
Results: INBRX-101 was well tolerated. Most treatment-emergent adverse events were grade ≤2. In part 2 (n=18; each dose, n=6), dose-related increases in serum functional AAT (fAAT) were observed; mean fAAT levels remained above the 21 µM target for up to 4 weeks after the final dose in the 120-mg/kg cohort. Antidrug antibodies had no meaningful impact on PK or PD. INBRX-101 was detected in pulmonary epithelial lining fluid (PELF) from all patients assessed (n=11), and PELF fAAT increased after dosing. PK/PD modeling projected steady-state serum fAAT ≥21µM at 120 mg/kg Q3W (average concentration ≈43µM; trough concentration ≈28µM) and Q4W (≈34µM; ≈21µM).
Conclusion: The favorable safety profile and ability to maintain serum fAAT levels >21µM with extended-interval dosing, support a phase 2 trial evaluating Q3W and Q4W dosing of INBRX-101.
Keywords: alpha-1 antitrypsin deficiency; augmentation therapy; clinical trials; orphan drugs; outcomes biologic agents.
JCOPDF © 2024.
Conflict of interest statement
MLB reports a relationship with the University of Florida College of Medicine that includes nonfinancial support. BTK reports a relationship with Takeda that includes consulting or advisory and speaking and lecture fees; a relationship with Boehringer Ingelheim that includes consulting or advisory fees; a relationship with Grifols that includes speaking and lecture fees; a relationship with Inhibrx, Inc., that includes consulting or advisory fees and travel reimbursement; and a relationship with the California Thoracic Society that includes nonfinancial support. HWF reports financial support provided by Hannibal Clinic Operations, LLC, and a relationship with Hannibal Clinic Operations, LLC, that includes employment. RM reports a relationship with Intellia Therapeutics, Inc., that includes consulting or advisory fees. CDB is an investigator for the ElevAATe trial (NCT05856331) that is sponsored by Inhibrx, Inc. MAC reports a relationship with Inhibrx, Inc., that includes consulting or advisory fees. EB reports a relationship with Inhibrx, Inc., that includes employment and equity or stocks. SB reports a relationship with Inhibrx, Inc., that includes employment and equity or stocks and has a patent titled “Effective Dosage of Recombinant Serpin-Fc Fusion Protein for Use in a Method of Treating AAT Deficiency in a Subject” pending to Inhibrx, Inc. TB reports a relationship with Inhibrx, Inc., that includes employment and equity or stocks. CV reports a relationship with Inhibrx, Inc., that includes employment and equity or stocks. VA reports a relationship with Inhibrx, Inc., that includes employment and equity or stocks. JK reports a relationship with Inhibrx, Inc., that includes employment and equity or stocks and reports a patent titled “Effective Dosage of Recombinant Serpin-Fc Fusion Protein for Use in a Method of Treating AAT Deficiency in a Subject” pending to Inhibrx, Inc. BE reports a relationship with Inhibrx, Inc., that includes employment and equity or stocks and reports patent #10,400,029 “Serpin Fusion Polypeptides and Methods of Use Thereof” issued to Inhibrx, Inc. AC, CLC, JEL, and AGV have nothing to disclose.
References
-
- Blanco I,Bueno P,Diego I,et al. Alpha-1 antitrypsin Pi*Z gene frequency and Pi*ZZ genotype numbers worldwide: an update. Int J Chron Obstruct Pulmon Dis. 2017;12:561-569. doi: https://doi.org/10.2147/COPD.S125389 - PMC - PubMed
-
- Sandhaus RA,Turino G,Brantly ML,et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis. 2016;3(3):668-682. doi: https://doi.org/10.15326/jcopdf.3.3.2015.0182 - PMC - PubMed
-
- de Serres FJ,Blanco I. Prevalence of alpha-1 antitrypsin deficiency alleles PI*S and PI*Z worldwide and effective screening for each of the five phenotypic classes PI*MS, PI*MZ, PI*SS, PI*SZ, and PI*ZZ: a comprehensive review. Ther Adv Respir Dis. 2012;6(5):277-295. doi: https://doi.org/10.1177/1753465812457113 - PubMed
-
- Craig TJ,Henao MP. Advances in managing COPD related to alpha-1 antitrypsin deficiency: an under-recognized genetic disorder. Allergy. 2018;73(11):2110-2121. doi: https://doi.org/10.1111/all.13558 - PMC - PubMed
-
- Strnad P,McElvaney NG,Lomas DA. Alpha-1 antitrypsin deficiency. N Engl J Med. 2020;382(15):1443-1455. doi: https://doi.org/10.1056/NEJMra1910234 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous