In vivo cisplatin-resistant neuroblastoma metastatic model reveals tumour necrosis factor receptor superfamily member 4 (TNFRSF4) as an independent prognostic factor of survival in neuroblastoma
- PMID: 38809883
- PMCID: PMC11135766
- DOI: 10.1371/journal.pone.0303643
In vivo cisplatin-resistant neuroblastoma metastatic model reveals tumour necrosis factor receptor superfamily member 4 (TNFRSF4) as an independent prognostic factor of survival in neuroblastoma
Abstract
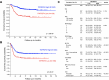
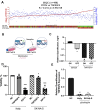
Neuroblastoma is the most common solid extracranial tumour in children. Despite major advances in available therapies, children with drug-resistant and/or recurrent neuroblastoma have a dismal outlook with 5-year survival rates of less than 20%. Therefore, tackling relapsed tumour biology by developing and characterising clinically relevant models is a priority in finding targetable vulnerability in neuroblastoma. Using matched cisplatin-sensitive KellyLuc and resistant KellyCis83Luc cell lines, we developed a cisplatin-resistant metastatic MYCN-amplified neuroblastoma model. The average number of metastases per mouse was significantly higher in the KellyCis83Luc group than in the KellyLuc group. The vast majority of sites were confirmed as having lymph node metastasis. Their stiffness characteristics of lymph node metastasis values were within the range reported for the patient samples. Targeted transcriptomic profiling of immuno-oncology genes identified tumour necrosis factor receptor superfamily member 4 (TNFRSF4) as a significantly dysregulated MYCN-independent gene. Importantly, differential TNFRSF4 expression was identified in tumour cells rather than lymphocytes. Low TNFRSF4 expression correlated with poor prognostic indicators in neuroblastoma, such as age at diagnosis, stage, and risk stratification and significantly associated with reduced probability of both event-free and overall survival in neuroblastoma. Therefore, TNFRSF4 Low expression is an independent prognostic factor of survival in neuroblastoma.
Copyright: © 2024 Murphy et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that they have no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures