Effects of post-saccadic oscillations on visual processing times
- PMID: 38809939
- PMCID: PMC11135737
- DOI: 10.1371/journal.pone.0302459
Effects of post-saccadic oscillations on visual processing times
Abstract
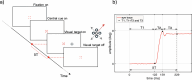
Saccadic eye movements enable us to search for the target of interest in a crowded scene or, in the case of goal-directed saccades, to simply bring the image of the peripheral target to the very centre of the fovea. This mechanism extends the use of the superior image processing performance of the fovea over a large visual field. We know that visual information is processed quickly at the end of each saccade but estimates of the times involved remain controversial. This study aims to investigate the processing of visual information during post fixation oscillations of the eyeball. A new psychophysical test measures the combined eye movement response latencies, including fixation duration and visual processing times. When the test is used in conjunction with an eye tracker, each component that makes up the 'integrated saccade latency' time, from the onset of the peripheral stimulus to the correct interpretation of the information carried by the stimulus, can be measured and the discrete components delineated. The results show that the time required to process and encode the stimulus attribute of interest at the end of a saccade is longer than the time needed to carry out the same task in the absence of an eye movement. We propose two principal hypotheses, each of which can account for this finding. 1. The known inhibition of afferent retinal signals during fast eye movements extends beyond the end point of the saccade. 2. The extended visual processing times measured when saccades are involved are caused by the transient loss of spatial resolution due to eyeball instability during post-saccadic oscillations. The latter can best be described as retinal image smear with greater loss of spatial resolution expected for stimuli of low luminance contrast.
Copyright: © 2024 Llapashtica et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Correlates of motor planning and postsaccadic fixation in the macaque monkey lateral geniculate nucleus.Exp Brain Res. 2006 Jan;168(1-2):62-75. doi: 10.1007/s00221-005-0093-z. Epub 2005 Sep 7. Exp Brain Res. 2006. PMID: 16151777
-
Saccadic "inhibition" unveils the late influence of image content on oculomotor programming.Exp Brain Res. 2024 Oct;242(10):2281-2294. doi: 10.1007/s00221-024-06890-z. Epub 2024 Jul 30. Exp Brain Res. 2024. PMID: 39080097 Free PMC article.
-
Low-Level Visual Information Is Maintained across Saccades, Allowing for a Postsaccadic Handoff between Visual Areas.J Neurosci. 2020 Dec 2;40(49):9476-9486. doi: 10.1523/JNEUROSCI.1169-20.2020. Epub 2020 Oct 28. J Neurosci. 2020. PMID: 33115930 Free PMC article.
-
Trans-retinal predictive signals of visual features are precise, saccade-specific and operate over a wide range of spatial frequencies.J Neurophysiol. 2024 Dec 1;132(6):1887-1895. doi: 10.1152/jn.00364.2024. Epub 2024 Nov 12. J Neurophysiol. 2024. PMID: 39531342
-
Shortening and prolongation of saccade latencies following microsaccades.Exp Brain Res. 2006 Mar;169(3):369-76. doi: 10.1007/s00221-005-0148-1. Epub 2005 Nov 23. Exp Brain Res. 2006. PMID: 16328308
Cited by
-
Biomechanical simulations of crystalline lens oscillations resulting from the changes in the gaze in an accommodated eye.Front Bioeng Biotechnol. 2025 Mar 5;13:1504769. doi: 10.3389/fbioe.2025.1504769. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40110498 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources

