Presynaptic nanoscale components of retrograde synaptic signaling
- PMID: 38809980
- PMCID: PMC11135421
- DOI: 10.1126/sciadv.ado0077
Presynaptic nanoscale components of retrograde synaptic signaling
Abstract
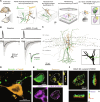
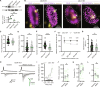
While our understanding of the nanoscale architecture of anterograde synaptic transmission is rapidly expanding, the qualitative and quantitative molecular principles underlying distinct mechanisms of retrograde synaptic communication remain elusive. We show that a particular form of tonic cannabinoid signaling is essential for setting target cell-dependent synaptic variability. It does not require the activity of the two major endocannabinoid-producing enzymes. Instead, by developing a workflow for physiological, anatomical, and molecular measurements at the same unitary synapse, we demonstrate that the nanoscale stoichiometric ratio of type 1 cannabinoid receptors (CB1Rs) to the release machinery is sufficient to predict synapse-specific release probability. Accordingly, selective decrease of extrasynaptic CB1Rs does not affect synaptic transmission, whereas in vivo exposure to the phytocannabinoid Δ9-tetrahydrocannabinol disrupts the intrasynaptic nanoscale stoichiometry and reduces synaptic variability. These findings imply that synapses leverage the nanoscale stoichiometry of presynaptic receptor coupling to the release machinery to establish synaptic strength in a target cell-dependent manner.
Figures





References
-
- Choquet D., Sainlos M., Sibarita J.-B., Advanced imaging and labelling methods to decipher brain cell organization and function. Nat. Rev. Neurosci. 22, 237–255 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

