CLUH maintains functional mitochondria and translation in motoneuronal axons and prevents peripheral neuropathy
- PMID: 38809982
- PMCID: PMC11135423
- DOI: 10.1126/sciadv.adn2050
CLUH maintains functional mitochondria and translation in motoneuronal axons and prevents peripheral neuropathy
Abstract
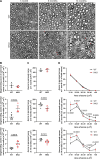
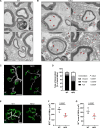
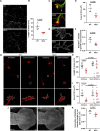
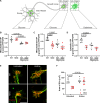
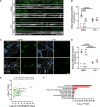
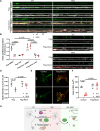
Transporting and translating mRNAs in axons is crucial for neuronal viability. Local synthesis of nuclear-encoded mitochondrial proteins protects long-lived axonal mitochondria from damage; however, the regulatory factors involved are largely unknown. We show that CLUH, which binds mRNAs encoding mitochondrial proteins, prevents peripheral neuropathy and motor deficits in the mouse. CLUH is enriched in the growth cone of developing spinal motoneurons and is required for their growth. The lack of CLUH affects the abundance of target mRNAs and the corresponding mitochondrial proteins more prominently in axons, leading to ATP deficits in the growth cone. CLUH interacts with ribosomal subunits, translation initiation, and ribosome recycling components and preserves axonal translation. Overexpression of the ribosome recycling factor ABCE1 rescues the mRNA and translation defects, as well as the growth cone size, in CLUH-deficient motoneurons. Thus, we demonstrate a role for CLUH in mitochondrial quality control and translational regulation in axons, which is essential for their development and long-term integrity and function.
Figures



References
-
- Harbauer A. B., Mitochondrial health maintenance in axons. Biochem. Soc. Trans. 45, 1045–1052 (2017). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

