Long-Term Effectiveness of Brodalumab for the Treatment of Moderate-To-Severe Psoriasis: A Real-Life Multicenter Study of Up to 3 Years in a Real-Life Italian Cohort
- PMID: 38810071
- PMCID: PMC11135952
- DOI: 10.5826/dpc.1402a152
Long-Term Effectiveness of Brodalumab for the Treatment of Moderate-To-Severe Psoriasis: A Real-Life Multicenter Study of Up to 3 Years in a Real-Life Italian Cohort
Abstract
Introduction: Data about the long-term effectiveness of brodalumab could be valuable in assessing patient adherence to treatment and improving psoriasis management.
Objective: The aim of our study was to evaluate the drug survival of brodalumab and identify any predictive factors for discontinuation.
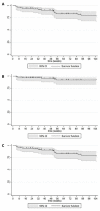
Methods: A multicenter retrospective study was conducted in patients with moderate-to-severe psoriasis who were treated for up to 3 years. We extracted data from patient files, related to the characteristics of the patients and the disease. Drug survival analysis was descriptively analyzed using Kaplan-Meier survival curves. Univariable and multivariable analyses were performed to assess baseline patient characteristics that predicted clinical response.
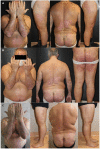
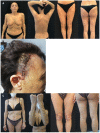
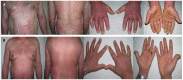
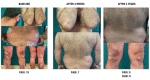
Results: The study included 90 patients. Among them, 28 (31.1%) suspended brodalumab through the observation period. At weeks 52, 104 and 156 the median PASI score were 0.0 [0.0 - 0.8], 0.0 [0.0 - 1.0] and 0.0 [0.0 - 0.0], respectively. The estimated cumulative survival rates at weeks 52 and 104 were 86.32% and 78.09%, respectively. In the multivariable survival analysis, predictor factors for overall discontinuation included body mass index (BMI) (OR 1.10, 95% CI 1.03 - 1.18), baseline PASI (OR 1.06, 95% CI 1.02 - 1.10), and psoriatic arthritis (OR 5.05, 95% CI 0.89 - 13.50).
Conclusions: Brodalumab has shown long-term effectiveness for up to 3 years. Considering baseline disease severity and patient characteristics could aid in optimizing the long-term management of psoriasis.
Conflict of interest statement
Figures
References
-
- Blair HA. Brodalumab: a review in moderate to severe plaque psoriasis. Drugs. 2018;78(4):495–504. - PubMed
-
- Russell CB, Rand H. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192(8):3828–3836. - PubMed
-
- Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, Toth D, Langley RG, Cather J, Gottlieb AB, Thaçi D, Krueger JG, Russell CB, Milmont CE, Li J, Klekotka PA, Kricorian G, Nirula A. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016 Aug;175(2):273–86. doi: 10.1111/bjd.14493. Epub 2016 Jun 23. - DOI - PubMed
-
- Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, Papp K, Spelman L, Toth D, Kerdel F, Armstrong AW, Stingl G, Kimball AB, Bachelez H, Wu JJ, Crowley J, Langley RG, Blicharski T, Paul C, Lacour JP, Tyring S, Kircik L, Chimenti S, Callis Duffin K, Bagel J, Koo J, Aras G, Li J, Song W, Milmont CE, Shi Y, Erondu N, Klekotka P, Kotzin B, Nirula A. Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. N Engl J Med. 2015 Oct;373(14):1318–28. doi: 10.1056/NEJMoa1503824. - DOI - PubMed
-
- Puig L, Lebwohl M, Bachelez H, Sobell J, Jacobson AA. Long-term efficacy and safety of brodalumab in the treatment of psoriasis: 120-week results from the randomized, double-blind, placebo- and active comparator-controlled phase 3 AMAGINE-2 trial. J Am Acad Dermatol. 2020 Feb;82(2):352–359. doi: 10.1016/j.jaad.2019.05.095. Epub 2019 Jun 5. - DOI - PubMed
LinkOut - more resources
Full Text Sources
