Exploring Defect-Engineered Metal-Organic Frameworks with 1,2,4-Triazolyl Isophthalate and Benzoate Linkers
- PMID: 38810089
- PMCID: PMC11167641
- DOI: 10.1021/acs.inorgchem.4c01589
Exploring Defect-Engineered Metal-Organic Frameworks with 1,2,4-Triazolyl Isophthalate and Benzoate Linkers
Abstract
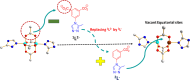
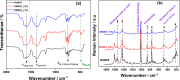
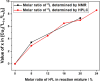
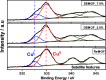
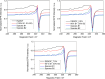
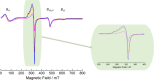
Synthesis and characterization of DEMOFs (defect-engineered metal-organic frameworks) with coordinatively unsaturated sites (CUSs) for gas adsorption, catalysis, and separation are reported. We use the mixed-linker approach to introduce defects in Cu2-paddle wheel units of MOFs [Cu2(Me-trz-ia)2] by replacing up to 7% of the 3-methyl-triazolyl isophthalate linker (1L2-) with the "defective linker" 3-methyl-triazolyl m-benzoate (2L-), causing uncoordinated equatorial sites. PXRD of DEMOFs shows broadened reflections; IR and Raman analysis demonstrates only marginal changes as compared to the regular MOF (ReMOF, without a defective linker). The concentration of the integrated defective linker in DEMOFs is determined by 1H NMR and HPLC, while PXRD patterns reveal that DEMOFs maintain phase purity and crystallinity. Combined XPS (X-ray photoelectron spectroscopy) and cw EPR (continuous wave electron paramagnetic resonance) spectroscopy analyses provide insights into the local structure of defective sites and charge balance, suggesting the presence of two types of defects. Notably, an increase in CuI concentration is observed with incorporation of defective linkers, correlating with the elevated isosteric heat of adsorption (ΔHads). Overall, this approach offers valuable insights into the creation and evolution of CUSs within MOFs through the integration of defective linkers.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Janiak C.; Vieth J. K. MOFs, MILs and More: Concepts, Properties and Applications for Porous Coordination Networks (PCNs). New J. Chem. 2010, 34, 2366–2388. 10.1039/c0nj00275e. - DOI
LinkOut - more resources
Full Text Sources

