The Role of Interferon-γ in Autoimmune Polyendocrine Syndrome Type 1
- PMID: 38810185
- PMCID: PMC11323209
- DOI: 10.1056/NEJMoa2312665
The Role of Interferon-γ in Autoimmune Polyendocrine Syndrome Type 1
Abstract
Background: Autoimmune polyendocrine syndrome type 1 (APS-1) is a life-threatening, autosomal recessive syndrome caused by autoimmune regulator (AIRE) deficiency. In APS-1, self-reactive T cells escape thymic negative selection, infiltrate organs, and drive autoimmune injury. The effector mechanisms governing T-cell-mediated damage in APS-1 remain poorly understood.
Methods: We examined whether APS-1 could be classified as a disease mediated by interferon-γ. We first assessed patients with APS-1 who were participating in a prospective natural history study and evaluated mRNA and protein expression in blood and tissues. We then examined the pathogenic role of interferon-γ using Aire-/-Ifng-/- mice and Aire-/- mice treated with the Janus kinase (JAK) inhibitor ruxolitinib. On the basis of our findings, we used ruxolitinib to treat five patients with APS-1 and assessed clinical, immunologic, histologic, transcriptional, and autoantibody responses.
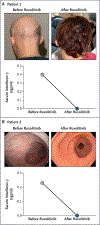
Results: Patients with APS-1 had enhanced interferon-γ responses in blood and in all examined autoimmunity-affected tissues. Aire-/- mice had selectively increased interferon-γ production by T cells and enhanced interferon-γ, phosphorylated signal transducer and activator of transcription 1 (pSTAT1), and CXCL9 signals in multiple organs. Ifng ablation or ruxolitinib-induced JAK-STAT blockade in Aire-/- mice normalized interferon-γ responses and averted T-cell infiltration and damage in organs. Ruxolitinib treatment of five patients with APS-1 led to decreased levels of T-cell-derived interferon-γ, normalized interferon-γ and CXCL9 levels, and remission of alopecia, oral candidiasis, nail dystrophy, gastritis, enteritis, arthritis, Sjögren's-like syndrome, urticaria, and thyroiditis. No serious adverse effects from ruxolitinib were identified in these patients.
Conclusions: Our findings indicate that APS-1, which is caused by AIRE deficiency, is characterized by excessive, multiorgan interferon-γ-mediated responses. JAK inhibition with ruxolitinib in five patients showed promising results. (Funded by the National Institute of Allergy and Infectious Diseases and others.).
Copyright © 2024 Massachusetts Medical Society.
Figures




References
-
- Oftedal BE, Hellesen A, Erichsen MM, et al. Dominant mutations in the autoimmune regulator AIRE are associated with common organ-specific autoimmune diseases. Immunity 2015;42:1185–96. - PubMed
-
- Ahonen P, Myllärniemi S, Sipilä I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med 1990;322:1829–36. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
