Sex-based differences in persistent lung inflammation following influenza infection of juvenile outbred mice
- PMID: 38810239
- PMCID: PMC11687968
- DOI: 10.1152/ajplung.00407.2023
Sex-based differences in persistent lung inflammation following influenza infection of juvenile outbred mice
Abstract
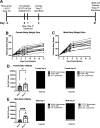
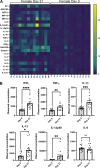
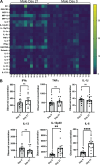
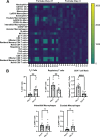
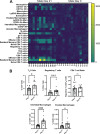
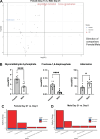
Children are susceptible to influenza infections and can experience severe disease presentation due to a lack of or limited pre-existing immunity. Despite the disproportionate impact influenza has on this population, there is a lack of focus on pediatric influenza research, particularly when it comes to identifying the pathogenesis of long-term outcomes that persist beyond the point of viral clearance. In this study, juvenile outbred male and female mice were infected with influenza and analyzed following viral clearance to determine how sex impacts the persistent inflammatory responses to influenza. It was found that females maintained a broader cytokine response in the lung following clearance of influenza, with innate, type I and type II cytokine signatures in almost all mice. Males, on the other hand, had higher levels of IL-6 and other macrophage-related cytokines, but no evidence of a type I or type II response. The immune landscape was similar in the lungs between males and females postinfection, but males had a higher regulatory T cell to TH1 ratio compared with female mice. Cytokine production positively correlated with the frequency of TH1 cells and exudate macrophages, as well as the number of cells in the bronchoalveolar lavage fluid. Furthermore, female lungs were enriched for metabolites involved in the glycolytic pathway, suggesting glycolysis is higher in female lungs compared with males after viral clearance. These data suggest juvenile female mice have persistent and excessive lung inflammation beyond the point of viral clearance, whereas juvenile males had a more immunosuppressive phenotype.NEW & NOTEWORTHY This study identifies sex-based differences in persistent lung inflammation following influenza infection in an outbred, juvenile animal model of pediatric infection. These findings indicate the importance of considering sex and age as variable in infectious disease research.
Keywords: T cell; cytokine; macrophage; pediatrics; pneumonia.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Ciancanelli MJ, Huang SX, Luthra P, Garner H, Itan Y, Volpi S, Lafaille FG, Trouillet C, Schmolke M, Albrecht RA, Israelsson E, Lim HK, Casadio M, Hermesh T, Lorenzo L, Leung LW, Pedergnana V, Boisson B, Okada S, Picard C, Ringuier B, Troussier F, Chaussabel D, Abel L, Pellier I, Notarangelo LD, García-Sastre A, Basler CF, Geissmann F, Zhang SY, Snoeck HW, Casanova JL. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science 348: 448–453, 2015. doi: 10.1126/science.aaa1578. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

