Wireless monitoring of respiration with EEG reveals relationships between respiration, behavior, and brain activity in freely moving mice
- PMID: 38810259
- PMCID: PMC11383384
- DOI: 10.1152/jn.00330.2023
Wireless monitoring of respiration with EEG reveals relationships between respiration, behavior, and brain activity in freely moving mice
Abstract
Active sampling in the olfactory domain is a fundamental aspect of mouse behavior, and there is increasing evidence that respiration-entrained neural activity outside of the olfactory system sets an important global brain rhythm. It is therefore crucial to accurately measure breathing during natural behaviors. We develop a new approach to do this in freely moving animals, by implanting a telemetry-based pressure sensor into the right jugular vein, which allows for wireless monitoring of thoracic pressure. After verifying this technique against standard head-fixed respiration measurements, we combined it with EEG and EMG recording and used evolving partial coherence analysis to investigate the relationship between respiration and brain activity across a range of experiments in which the mice could move freely. During voluntary exploration of odors and objects, we found that the association between respiration and cortical activity in the delta and theta frequency range decreased, whereas the association between respiration and cortical activity in the alpha range increased. During sleep, however, the presentation of an odor was able to cause a transient increase in sniffing without changing dominant sleep rhythms (delta and theta) in the cortex. Our data align with the emerging idea that the respiration rhythm could act as a synchronizing scaffold for specific brain rhythms during wakefulness and exploration, but suggest that respiratory changes are less able to impact brain activity during sleep. Combining wireless respiration monitoring with different types of brain recording across a variety of behaviors will further increase our understanding of the important links between active sampling, passive respiration, and neural activity.NEW & NOTEWORTHY Animals can alter their respiration rate to actively sample their environment, and increasing evidence suggests that neurons across the brain align their firing to this changing rhythm. We developed a new approach to measure sniffing in freely moving mice while simultaneously recording brain activity, and uncovered how specific cortical rhythms changed their coherence with respiration rhythm during natural behaviors and across arousal states.
Keywords: EEG; exploration; respiration; sleep.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

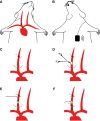

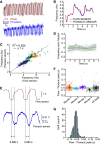
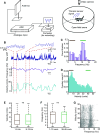
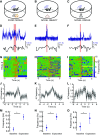


Similar articles
-
Hippocampal Respiration-Driven Rhythm Distinct from Theta Oscillations in Awake Mice.J Neurosci. 2016 Jan 6;36(1):162-77. doi: 10.1523/JNEUROSCI.2848-15.2016. J Neurosci. 2016. PMID: 26740658 Free PMC article.
-
Polygraphic Recording Procedure for Measuring Sleep in Mice.J Vis Exp. 2016 Jan 25;(107):e53678. doi: 10.3791/53678. J Vis Exp. 2016. PMID: 26863349 Free PMC article.
-
[An EEG study of different behavioral states of freely moving dolphins].Zh Vyssh Nerv Deiat Im I P Pavlova. 1975 Mar-Apr;25(2):396-401. Zh Vyssh Nerv Deiat Im I P Pavlova. 1975. PMID: 1210706 Russian.
-
[Selective stimulations and lesions of the rat brain nuclei as the models for research of the human sleep pathology mechanisms].Glas Srp Akad Nauka Med. 2011;(51):85-97. Glas Srp Akad Nauka Med. 2011. PMID: 22165729 Review. Serbian.
-
Counterpointing the functional role of the forebrain and of the brainstem in the control of the sleep-waking system.J Sleep Res. 2004 Sep;13(3):179-208. doi: 10.1111/j.1365-2869.2004.00412.x. J Sleep Res. 2004. PMID: 15339255 Review.
Cited by
-
Surgical Implantation of a Telemetry-Based Pressure Sensor in the Internal Jugular Vein to Monitor Respiration Wirelessly.Bio Protoc. 2025 Jun 20;15(12):e5343. doi: 10.21769/BioProtoc.5343. eCollection 2025 Jun 20. Bio Protoc. 2025. PMID: 40657540 Free PMC article.
-
Dynamics of odor-source localization: Insights from real-time odor plume recordings and head-motion tracking in freely moving mice.PLoS One. 2024 Sep 26;19(9):e0310254. doi: 10.1371/journal.pone.0310254. eCollection 2024. PLoS One. 2024. PMID: 39325742 Free PMC article.
-
Olfactory neurofeedback: current state and possibilities for further development.Front Hum Neurosci. 2024 Nov 29;18:1419552. doi: 10.3389/fnhum.2024.1419552. eCollection 2024. Front Hum Neurosci. 2024. PMID: 39677402 Free PMC article.
-
Dynamics of odor-source localization: Insights from real-time odor plume recordings and head-motion tracking in freely moving mice.bioRxiv [Preprint]. 2024 Jul 5:2023.11.10.566539. doi: 10.1101/2023.11.10.566539. bioRxiv. 2024. Update in: PLoS One. 2024 Sep 26;19(9):e0310254. doi: 10.1371/journal.pone.0310254. PMID: 38014041 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

