FOXO1 regulates RUNX2 ubiquitination through SMURF2 in calcific aortic valve disease
- PMID: 38810422
- PMCID: PMC11167395
- DOI: 10.1016/j.redox.2024.103215
FOXO1 regulates RUNX2 ubiquitination through SMURF2 in calcific aortic valve disease
Abstract
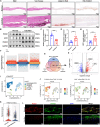
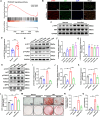
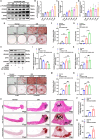
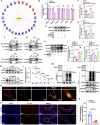
The prevalence of calcific aortic valve disease (CAVD) remains substantial while there is currently no medical therapy available. Forkhead box O1 (FOXO1) is known to be involved in the pathogenesis of cardiovascular diseases, including vascular calcification and atherosclerosis; however, its specific role in calcific aortic valve disease remains to be elucidated. In this study, we identified FOXO1 significantly down-regulated in the aortic valve interstitial cells (VICs) of calcified aortic valves by investigating clinical specimens and GEO database analysis. FOXO1 silencing or inhibition promoted VICs osteogenic differentiation in vitro and aortic valve calcification in Apoe-/- mice, respectively. We identified that FOXO1 facilitated the ubiquitination and degradation of RUNX2, which process was mainly mediated by SMAD-specific E3 ubiquitin ligase 2 (SMURF2). Our discoveries unveil a heretofore unacknowledged mechanism involving the FOXO1/SMURF2/RUNX2 axis in CAVD, thereby proposing the potential therapeutic utility of FOXO1 or SMURF2 as viable strategies to impede the progression of CAVD.
Keywords: Calcific aortic valve disease; Forkhead box O1; Phosphorylation; SMAD-Specific E3 ubiquitin ligase 2; Ubiquitination.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Nkomo V.T., Gardin J.M., Skelton T.N., Gottdiener J.S., Scott C.G., Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011. - PubMed
-
- Coffey S., Cox B., Williams M.J.A. The prevalence, incidence, progression, and risks of aortic valve sclerosis. J. Am. Coll. Cardiol. 2014;63(25):2852–2861. - PubMed
-
- Yadgir S., Johnson C.O., Aboyans V., Adebayo O.M., Adedoyin R.A., Afarideh M., et al. Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990–2017. Circulation. 2020;141(21):1670–1680. - PubMed
-
- Goody P.R., Hosen M.R., Christmann D., Niepmann S.T., Zietzer A., Adam M., et al. Aortic valve stenosis. Arterioscler. Thromb. Vasc. Biol. 2020;40(4):885–900. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

