GAPDH inhibition mediated by thiol oxidation in human airway epithelial cells exposed to an environmental peroxide
- PMID: 38810423
- PMCID: PMC11167385
- DOI: 10.1016/j.redox.2024.103199
GAPDH inhibition mediated by thiol oxidation in human airway epithelial cells exposed to an environmental peroxide
Abstract
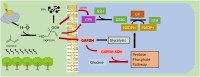
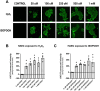
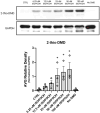
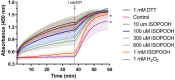
Intracellular redox homeostasis in the airway epithelium is closely regulated through adaptive signaling and metabolic pathways. However, inhalational exposure to xenobiotic stressors such as secondary organic aerosols (SOA) can alter intracellular redox homeostasis. Isoprene hydroxy hydroperoxide (ISOPOOH), a ubiquitous volatile organic compound derived from the atmospheric photooxidation of biogenic isoprene, is a major contributor to SOA. We have previously demonstrated that exposure of human airway epithelial cells (HAEC) to ISOPOOH induces oxidative stress through multiple mechanisms including lipid peroxidation, glutathione oxidation, and alterations of glycolytic metabolism. Using dimedone-based reagents and copper catalyzed azo-alkynyl cycloaddition to tag intracellular protein thiol oxidation, we demonstrate that exposure of HAEC to micromolar levels of ISOPOOH induces reversible oxidation of cysteinyl thiols in multiple intracellular proteins, including GAPDH, that was accompanied by a dose-dependent loss of GAPDH enzymatic activity. These results demonstrate that ISOPOOH induces an oxidative modification of intracellular proteins that results in loss of GAPDH activity, which ultimately impacts the dynamic regulation of the intracellular redox homeostatic landscape in HAEC.
Keywords: Air pollution; Cellular bioenergetics; GAPDH (glyceraldehyde-3-phosphate dehydrogenase); Oxidative stress; Protein thiol oxidation; Secondary organic aerosols.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Pandis S.N., Harley R.A., Cass G.R., Seinfeld J.H. Secondary organic aerosol formation and transport. Atmos. Environ., Part A. 1992;26:2269–2282. doi: 10.1016/0960-1686(92)90358-R. - DOI
-
- Kramer A.J., Rattanavaraha W., Zhang Z., Gold A., Surratt J.D., Lin Y.-H. Assessing the oxidative potential of isoprene-derived epoxides and secondary organic aerosol | Enhanced Reader. Atmos. Environ. 2016;130:211–218.
-
- Arashiro M., Lin Y.H., Sexton K.G., Zhang Z., Jaspers I., Fry R.C., Vizuete W.G., Gold A., Surratt J.D. In vitro exposure to isoprene-derived secondary organic aerosol by direct deposition and its effects on COX-2 and IL-8 gene expression. Atmos. Chem. Phys. 2016;16:14079–14090. doi: 10.5194/acp-16-14079-2016. - DOI
-
- Lin Y.-H.H., Arashiro M., Clapp P.W., Cui T., Sexton K.G., Vizuete W., Gold A., Jaspers I., Fry R.C., Surratt J.D. Gene expression profiling in human lung cells exposed to isoprene-derived secondary organic aerosol. Environ. Sci. Technol. 2017;51:8166–8175. doi: 10.1021/acs.est.7b01967. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

