Specificity profiling of deubiquitylases against endogenously generated ubiquitin-protein conjugates
- PMID: 38810651
- PMCID: PMC11260241
- DOI: 10.1016/j.chembiol.2024.05.001
Specificity profiling of deubiquitylases against endogenously generated ubiquitin-protein conjugates
Abstract
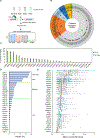
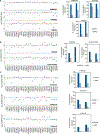
Deubiquitylating enzymes (DUBs) remove ubiquitin from proteins thereby regulating their stability or activity. Our understanding of DUB-substrate specificity is limited because DUBs are typically not compared to each other against many physiological substrates. By broadly inhibiting DUBs in Xenopus egg extract, we generated hundreds of ubiquitylated proteins and compared the ability of 30 DUBs to deubiquitylate them using quantitative proteomics. We identified five high-impact DUBs (USP7, USP9X, USP36, USP15, and USP24) that each reduced ubiquitylation of over 10% of the isolated proteins. Candidate substrates of high-impact DUBs showed substantial overlap and were enriched for disordered regions, suggesting this feature may promote substrate recognition. Other DUBs showed lower impact and non-overlapping specificity, targeting distinct non-disordered proteins including complexes such as the ribosome or the proteasome. Altogether our study identifies candidate DUB substrates and defines patterns of functional redundancy and specificity, revealing substrate characteristics that may influence DUB-substrate recognition.
Keywords: DUB substrates; TMT-proteomics; Xenopus; deubiquitylating enzymes; ubiquitin.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Update of
-
Substrate identification and specificity profiling of deubiquitylases against endogenously-generated ubiquitin-protein conjugates.bioRxiv [Preprint]. 2023 Dec 20:2023.12.20.572581. doi: 10.1101/2023.12.20.572581. bioRxiv. 2023. Update in: Cell Chem Biol. 2024 Jul 18;31(7):1349-1362.e5. doi: 10.1016/j.chembiol.2024.05.001. PMID: 38187689 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

