Hemophagocytic Lymphohistiocytosis Gene Variants in Severe Aplastic Anemia and Their Impact on Hematopoietic Cell Transplantation Outcomes
- PMID: 38810947
- PMCID: PMC11296907
- DOI: 10.1016/j.jtct.2024.05.017
Hemophagocytic Lymphohistiocytosis Gene Variants in Severe Aplastic Anemia and Their Impact on Hematopoietic Cell Transplantation Outcomes
Abstract
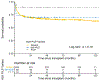
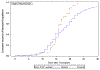
Germline genetic testing for patients with severe aplastic anemia (SAA) is recommended to guide treatment, including the use of immunosuppressive therapy and/or adjustment of hematopoietic cell transplantation (HCT) modalities. Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening hyperinflammatory condition often associated with cytopenias with autosomal recessive (AR) or X-linked recessive (XLR) inheritance. HLH is part of the SAA differential diagnosis, and genetic testing may identify variants in HLH genes in patients with SAA. The impact of pathogenic/likely pathogenic (P/LP) variants in HLH genes on HCT outcomes in SAA is unclear. In this study, we aimed to determine the frequency of HLH gene variants in a large cohort of patients with acquired SAA and to evaluate their association(s) with HCT outcomes. The Transplant Outcomes in Aplastic Anemia project, a collaboration between the National Cancer Institute and the Center for International Blood and Marrow Transplant Research, collected genomic and clinical data from 824 patients who underwent HCT for SAA between 1989 and 2015. We excluded 140 patients with inherited bone marrow failure syndromes and used exome sequencing data from the remaining 684 patients with acquired SAA to identify P/LP variants in 14 HLH-associated genes (11 AR, 3 XLR) curated using American College of Medical Genetics and Genomics/Association of Molecular Pathology (ACMG/AMP) criteria. Deleterious variants of uncertain significance (del-VUS) were defined as those not meeting the ACMG/AMP P/LP criteria but with damaging predictions in ≥3 of 5 meta-predictors (BayesDel, REVEL, CADD, MetaSVM, and/or EIGEN). The Kaplan-Meier estimator was used to calculate the probability of overall survival (OS) after HCT, and the cumulative incidence calculator was used for other HCT outcomes, accounting for relevant competing risks. There were 46 HLH variants in 49 of the 684 patients (7.2%). Seventeen variants in 19 patients (2.8%) were P/LP; 8 of these were loss-of-function variants. Among the 19 patients with P/LP HLH variants, 16 (84%) had monoallelic variants in genes with AR inheritance, and 3 had variants in XLR genes. PRF1 was the most frequently affected gene (in 8 of the 19 patients). We found no statistically significant differences in transplantation-related factors between patients with and those without P/LP HLH variants. The 5-year survival probability was 89% (95% confidence interval [CI], 72% to 99%) in patients with P/LP HLH variants and 70% (95% CI, 53% to 85%) in those with del-VUS HLH variants, compared to 66% (95% CI, 62% to 70%) in those without variants (P = .16, log-rank test). The median time to neutrophil engraftment was 16 days for patients with P/LP HLH variants and 18 days in those with del-VUS HLH variants or without variants combined (P = .01, Gray's test). No statistically significant associations between P/LP HLH variants and the risk of acute or chronic graft-versus-host disease were noted. In this large cohort of patients with acquired SAA, we found that 2.8% of patients harbored a P/LP variant in an HLH gene. No negative effects of HLH gene variants on post-HCT survival were noted. The small number of patients with P/LP HLH variants limits the study's ability to provide conclusive evidence; nonetheless, our data suggest that there is no need for special transplantation considerations for patients with SAA carrying P/LP variants.
Keywords: Aplastic anemia; Hematopoietic cell transplantation; Hemophagocytic lymphohistiocytosis; PRF1; Whole exome sequencing.
Published by Elsevier Inc.
Figures
Similar articles
-
First-line allogeneic hematopoietic stem cell transplantation of HLA-matched sibling donors compared with first-line ciclosporin and/or antithymocyte or antilymphocyte globulin for acquired severe aplastic anemia.Cochrane Database Syst Rev. 2013 Jul 23;2013(7):CD006407. doi: 10.1002/14651858.CD006407.pub2. Cochrane Database Syst Rev. 2013. PMID: 23881658 Free PMC article.
-
Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults.Cochrane Database Syst Rev. 2014 Apr 20;2014(4):CD010189. doi: 10.1002/14651858.CD010189.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2024 Nov 7;11:CD010189. doi: 10.1002/14651858.CD010189.pub3. PMID: 24748537 Free PMC article. Updated.
-
Does Augmenting Irradiated Autografts With Free Vascularized Fibula Graft in Patients With Bone Loss From a Malignant Tumor Achieve Union, Function, and Complication Rate Comparably to Patients Without Bone Loss and Augmentation When Reconstructing Intercalary Resections in the Lower Extremity?Clin Orthop Relat Res. 2025 Jun 26. doi: 10.1097/CORR.0000000000003599. Online ahead of print. Clin Orthop Relat Res. 2025. PMID: 40569278
-
Upfront Haploidentical Hematopoietic Cell Transplantation Using αβ+ T Cell-Depleted Peripheral Blood Stem Cells for Pediatric Patients with Acquired Severe Aplastic Anemia.Transplant Cell Ther. 2025 Jun 10:S2666-6367(25)01255-2. doi: 10.1016/j.jtct.2025.06.012. Online ahead of print. Transplant Cell Ther. 2025. PMID: 40505813
-
Sex and gender as predictors for allograft and patient-relevant outcomes after kidney transplantation.Cochrane Database Syst Rev. 2024 Dec 19;12(12):CD014966. doi: 10.1002/14651858.CD014966.pub2. Cochrane Database Syst Rev. 2024. PMID: 39698949
Cited by
-
Severe Aplastic Anemia Complicated with Fatal Invasive Fungal Infections in a Young Patient Harboring Perforin Gene Polymorphisms.Hematol Rep. 2025 May 6;17(3):25. doi: 10.3390/hematolrep17030025. Hematol Rep. 2025. PMID: 40407635 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

