Ventricular Intramyocardial Navigation for Tachycardia Ablation Guided by Electrograms (VINTAGE): Deep Ablation in Inaccessible Targets
- PMID: 38811066
- PMCID: PMC11372842
- DOI: 10.1016/j.jacep.2024.04.002
Ventricular Intramyocardial Navigation for Tachycardia Ablation Guided by Electrograms (VINTAGE): Deep Ablation in Inaccessible Targets
Abstract
Background: Deep intramural ventricular tachycardia substrate targets are difficult to access, map, and ablate from endocardial and epicardial surfaces, resulting in high recurrence rates.
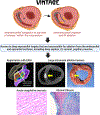
Objectives: In this study, the authors introduce a novel approach called ventricular intramyocardial navigation for tachycardia ablation guided by electrograms (VINTAGE) to access and ablate anatomically challenging ventricular tachycardia from within the myocardium.
Methods: Guidewire/microcatheter combinations were navigated deep throughout the extravascular myocardium, accessed directly from the right ventricle cavity, in Yorkshire swine (6 naive, 1 infarcted). Devices were steered to various intramyocardial targets including the left ventricle summit, guided by fluoroscopy, unipolar electrograms, and/or electroanatomic mapping. Radiofrequency ablations were performed to characterize ablation parameters and reproducibility. Intramyocardial saline irrigation began 1 minute before ablation and continued throughout. Lesions were analyzed on cardiac magnetic resonance and necropsy.
Results: VINTAGE was feasible in all animals within naive and infarcted myocardium. Forty-three lesions were created, using various guidewires and power settings. Forty-one (95%) lesions were detected on cardiac magnetic resonance and 38 (88%) on necropsy; all undetected lesions resulted from intentionally subtherapeutic ablation energy (10 W). Larger-diameter guidewires yielded larger size lesions. Lesion volumes on necropsy were significantly larger at 20 W than 10 W (178 mm3 [Q1-Q3: 104-382 mm3] vs 49 mm3 [Q1-Q3: 35-93 mm3]; P = 0.02). Higher power (30 W) did not create larger lesions. Median impedance dropped with preablation irrigation by 12 Ω (Q1-Q3: 8-17 Ω), followed by a further 15-Ω (Q1-Q3: 11-19 Ω) drop during ablation. Intramyocardial navigation, ablation, and irrigation were not associated with any complications.
Conclusions: VINTAGE was safe and effective at creating intramural ablation lesions in targets traditionally considered inaccessible from the endocardium and epicardium, both naive and infarcted. Intramyocardial guidewire irrigation and ablation at 20 W creates reproducibly large intramural lesions.
Keywords: arrhythmia; cardiac catheterization; cardiac electrophysiologic techniques; catheter ablation; electrocardiographic radial depth navigation; radiofrequency therapy; ventricular/diagnosis/physiopathology/therapy tachycardia.
Published by Elsevier Inc.
Conflict of interest statement
Funding Support and Author Disclosures Supported by the Division of Intramural Research, National Heart Lung and Blood Institute, National Institutes of Health (NIH), grant Z01-HL006040 (to Dr Lederman). Dr Halaby is a coinventor on applicable patent applications assigned to the NIH. Dr Bruce is a coinventor on applicable patent applications assigned to the NIH. Dr Kolandaivelu is a coinventor on applicable patent applications assigned to the NIH. Dr Rogers has performed consulting for Edwards Lifesciences, Medtronic, Boston Scientific, and Transmural Systems; has served on the Advisory Board for Medtronic and Boston Scientific; holds equity interest in Transmural Systems; and is a coinventor on patents, assigned to the NIH, for transcatheter electrosurgery devices. Dr Khan is a coinventor on applicable patent applications assigned to the NIH; has served as a proctor for Edwards Lifesciences and Medtronic; and holds equity in Transmural Systems. Dr Yildirim is a coinventor on applicable patent applications assigned to the NIH. Dr Lederman is a coinventor on applicable patent applications assigned to the NIH. Dr Babaliaros has served as a consultant for Edwards Lifesciences, Abbott Vascular, and Transmural Systems, and his employer has research contracts for the clinical investigation of transcatheter aortic and mitral devices from Edwards Lifesciences, Abbott Vascular, Medtronic, St Jude Medical, and Boston Scientific. Dr Greenbaum has served as a proctor for Edwards Lifesciences, Medtronic, and Abbott Vascular and holds equity in Transmural Systems and Excision Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures





References
-
- Dinov B, Fiedler L, Schönbauer R et al. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation 2014;129:728–36. - PubMed
-
- Muser D, Santangeli P, Castro SA et al. Long-Term Outcome After Catheter Ablation of Ventricular Tachycardia in Patients With Nonischemic Dilated Cardiomyopathy. Circ Arrhythm Electrophysiol 2016;9. - PubMed
-
- Nguyen DT, Olson M, Zheng L, Barham W, Moss JD, Sauer WH. Effect of Irrigant Characteristics on Lesion Formation After Radiofrequency Energy Delivery Using Ablation Catheters with Actively Cooled Tips. J Cardiovasc Electrophysiol 2015;26:792–8. - PubMed
-
- Shapira-Daniels A, Barkagan M, Rottmann M et al. Modulating the Baseline Impedance: An Adjunctive Technique for Maximizing Radiofrequency Lesion Dimensions in Deep and Intramural Ventricular Substrate: An Adjunctive Technique for Maximizing Radiofrequency Lesion Dimensions in Deep and Intramural Ventricular Substrate. Circ Arrhythm Electrophysiol 2019;12:e007336. - PMC - PubMed
-
- Chang RJ, Stevenson WG, Saxon LA, Parker J. Increasing catheter ablation lesion size by simultaneous application of radiofrequency current to two adjacent sites. Am Heart J 1993;125:1276–84. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

