Ablation of Fatty Acid Transport Protein-4 Enhances Cone Survival, M-cone Vision, and Synthesis of Cone-Tropic 9- cis-Retinal in rd 12 Mouse Model of Leber Congenital Amaurosis
- PMID: 38811164
- PMCID: PMC11223470
- DOI: 10.1523/JNEUROSCI.1994-23.2024
Ablation of Fatty Acid Transport Protein-4 Enhances Cone Survival, M-cone Vision, and Synthesis of Cone-Tropic 9- cis-Retinal in rd 12 Mouse Model of Leber Congenital Amaurosis
Abstract
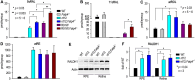
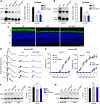
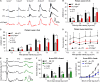
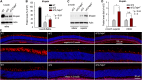
The canonical visual cycle employing RPE65 as the retinoid isomerase regenerates 11-cis-retinal to support both rod- and cone-mediated vision. Mutations of RPE65 are associated with Leber congenital amaurosis that results in rod and cone photoreceptor degeneration and vision loss of affected patients at an early age. Dark-reared Rpe65-/- mouse has been known to form isorhodopsin that employs 9-cis-retinal as the photosensitive chromophore. The mechanism regulating 9-cis-retinal synthesis and the role of the endogenous 9-cis-retinal in cone survival and function remain largely unknown. In this study, we found that ablation of fatty acid transport protein-4 (FATP4), a negative regulator of 11-cis-retinol synthesis catalyzed by RPE65, increased the formation of 9-cis-retinal, but not 11-cis-retinal, in a light-independent mechanism in both sexes of RPE65-null rd12 mice. Both rd12 and rd12;Fatp4-/- mice contained a massive amount of all-trans-retinyl esters in the eyes, exhibiting comparable scotopic vision and rod degeneration. However, expression levels of M- and S-opsins as well as numbers of M- and S-cones surviving in the superior retinas of rd12;Fatp4-/ - mice were at least twofold greater than those in age-matched rd12 mice. Moreover, FATP4 deficiency significantly shortened photopic b-wave implicit time, improved M-cone visual function, and substantially deaccelerated the progression of cone degeneration in rd12 mice, whereas FATP4 deficiency in mice with wild-type Rpe65 alleles neither induced 9-cis-retinal formation nor influenced cone survival and function. These results identify FATP4 as a new regulator of synthesis of 9-cis-retinal, which is a "cone-tropic" chromophore supporting cone survival and function in the retinas with defective RPE65.
Keywords: 9-cis-retinal; FATP4; RPE65 isomerase; cone photoreceptor; retinal dystrophy.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Inverse correlation between fatty acid transport protein 4 and vision in Leber congenital amaurosis associated with RPE65 mutation.Proc Natl Acad Sci U S A. 2020 Dec 15;117(50):32114-32123. doi: 10.1073/pnas.2012623117. Epub 2020 Nov 30. Proc Natl Acad Sci U S A. 2020. PMID: 33257550 Free PMC article.
-
Intraperitoneal chromophore injections delay early-onset and rapid retinal cone degeneration in a mouse model of Leber congenital amaurosis.Exp Eye Res. 2021 Nov;212:108776. doi: 10.1016/j.exer.2021.108776. Epub 2021 Sep 25. Exp Eye Res. 2021. PMID: 34582935
-
Pharmacological Amelioration of Cone Survival and Vision in a Mouse Model for Leber Congenital Amaurosis.J Neurosci. 2016 May 25;36(21):5808-19. doi: 10.1523/JNEUROSCI.3857-15.2016. J Neurosci. 2016. PMID: 27225770 Free PMC article.
-
Cone Health and Retinoids.Prog Mol Biol Transl Sci. 2015;134:465-76. doi: 10.1016/bs.pmbts.2015.06.002. Epub 2015 Jul 2. Prog Mol Biol Transl Sci. 2015. PMID: 26310171 Review.
-
Properties and Therapeutic Implications of an Enigmatic D477G RPE65 Variant Associated with Autosomal Dominant Retinitis Pigmentosa.Genes (Basel). 2020 Nov 27;11(12):1420. doi: 10.3390/genes11121420. Genes (Basel). 2020. PMID: 33261050 Free PMC article. Review.
References
-
- Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K (2004) Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem 279:10422–10432. 10.1074/jbc.M312410200 - DOI - PMC - PubMed
-
- Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, Nolan JM (2016) Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res 50:34–66. 10.1016/j.preteyeres.2015.10.003 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
